Published online by Cambridge University Press: 17 January 2013
The idea of chemical structure, as founded on that of atomicity (or the equivalence of atoms), enables us to divide any molecule, whose chemical structure is known, into radicals. The number of ways in which this may be done increases with the complexity of the molecule. Each of these modes of division corresponds to a series of conceivable reactions, some of which have been observed. Any one of these series may be made the basis of classification; but it is obviously most convenient to select for this purpose the most characteristic reactions, and those which are common to such substances as form natural groups. In studying these, we find that each series implies the presence of a particular radical, within which the reactions in question take place. We may call such series of reactions the Generic reactions, and the corresponding radicals Generic radicals. These are sometimes residues of double decomposition, but very frequently this is not the case, and this may account for the fact, that the importance of these generic radicals has been very much overlooked.
page 333 note * I had proposed to express the radical (COHO)' by the symbol ![]() before I was aware that Butlerow had already used the symbol A to represent the same radical. While fully acknowledging the priority of Butlerow's recognition of this radical, I prefer to retain the symbol
before I was aware that Butlerow had already used the symbol A to represent the same radical. While fully acknowledging the priority of Butlerow's recognition of this radical, I prefer to retain the symbol ![]() By using such of the Greek capitals as differ from the Roman in form, to represent generic radicals, we avoid the danger of confounding them with elementary atoms.
By using such of the Greek capitals as differ from the Roman in form, to represent generic radicals, we avoid the danger of confounding them with elementary atoms.
page 334 note * Translating Kekulé's graphic formula for phenylic alcohol into the system used in this paper, we have 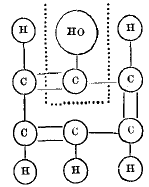 in which we have the triatomic radical C(HO) united to the triatomic radical C5H5.
in which we have the triatomic radical C(HO) united to the triatomic radical C5H5.
page 334 note † Using the symbol 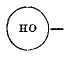 as a contraction for
as a contraction for ![]()
page 338 note * As this radical occurs very frequently, it may be advantageous to have a single symbol for it, and I have been in the habit of using the Greek letter Σ for this purpose. Thus we have ![]() acetic acid; CH3Σ methylsulphuric acid;
acetic acid; CH3Σ methylsulphuric acid; ![]() sulphacetic acid, &c.
sulphacetic acid, &c.
page 338 note † See Kolbé, , Lehrbuch der Organischen Chemie, Bd. ii. s. 742.Google Scholar