Published online by Cambridge University Press: 06 July 2012
The attempt to account for the behaviour of gases by attributing their apparently continuous pressure to exceedingly numerous, but nearly infinitesimal, impacts on the containing vessel is probably very old. It certainly occurs, with some little development, in Hooke's tract of 1676, Lectures de potentiâ restitutivâ, or of Spring; and, somewhat more fully developed, in the Hydrodynamica of D. Bernoulli, 1738. Traces of it are to be found in the writings of Le Sage and Prévost some 80 or 90 years ago. It was recalled to notice in 1847 by Herapath in his Mathematical Physics, and applied, in 1848, by Joule to the calculation of the average speed of the particles in a mass of hydrogen at various temperatures. Joule expressly states that his results are independent of the number of the particles, and of their directions of motion, as also of their mutual collisions.
page 65 note * The paper is reprinted Phil. Mag. 1857, II. See especially p. 215.
page 66 note * Compare another investigation, also by Clerk-Maxwell but based on Boltzmann's processes, which, is given in Nature, viii. 537 (Oct. 23, 1873). Some remarks on this will be made at the end of the paper. Meanwhile it is sufficient to point out that this, like the (less elaborate) investigations of Meyer and Watson, merely attempts to show that a certain state, once attained, is permanent. It gives no indication of the rate at which it would be restored if disturbed. As will be seen later, I think that this “rate” is an element of very great importance on account of the reasons for confidence (in the general results of the investigation) which it so strikingly furnishes.
page 77 note * Phil. Mag., 1860.
page 85 note * Prof. Cayley has called my attention, in connection with this, to the following expression from a Trinity (Cambridge) Examination Paper:—
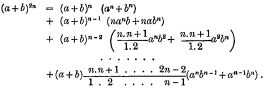
page 88 note * Chem. Soc. Jour., xiii. (1875), p. 493 Google Scholar.
page 90 note * Annales de Chimie, xxii. 1881 Google Scholar.
page 90 note † Phil. Mag., 1873, ii. 453 Google Scholar. See also Nature, viii. 298.
page 91 note * Nature, viii., May 29, 1873 Google Scholar. Maxwell's name does not occur in the Index to this volume, though he has made at least five contributions to it, most of which bear on the present subject:—viz. at pp. 85, 298, 361, 527, 537.