Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Stewart, A. D.
1994.
Possible sources of clastic sediment in the Late Proterozoic Torridon Group, from geochemical mass balance.
Transactions of the Royal Society of Edinburgh: Earth Sciences,
Vol. 85,
Issue. 4,
p.
303.
Bierman, Paul R.
Albrecht, Achim
Bothner, Michael H.
Brown, Erik T.
Bullen, Thomas D.
Gray, Leda Beth
and
Turpin, Laurent
1998.
Isotope Tracers in Catchment Hydrology.
p.
647.
2002.
References.
Geological Society, London, Memoirs,
Vol. 24,
Issue. 1,
p.
119.
Sensarma, S.
Rajamani, V.
and
Tripathi, Jayant K.
2008.
Petrography and geochemical characteristics of the sediments of the small River Hemavati, Southern India: Implications for provenance and weathering processes.
Sedimentary Geology,
Vol. 205,
Issue. 3-4,
p.
111.
Mishra, M.
and
Sen, S.
2011.
Geochemical signatures for the grain size variation in the siliciclastics of Kaimur Group, Vindhyan Supergroup from Markundi ghat, Sonbhadra district, (U.P.), India.
Geochemistry International,
Vol. 49,
Issue. 3,
p.
274.
McLennan, Scott M.
2012.
Sedimentary Geology of Mars.
p.
119.
McLennan, S. M.
Anderson, R. B.
Bell, J. F.
Bridges, J. C.
Calef, F.
Campbell, J. L.
Clark, B. C.
Clegg, S.
Conrad, P.
Cousin, A.
Des Marais, D. J.
Dromart, G.
Dyar, M. D.
Edgar, L. A.
Ehlmann, B. L.
Fabre, C.
Forni, O.
Gasnault, O.
Gellert, R.
Gordon, S.
Grant, J. A.
Grotzinger, J. P.
Gupta, S.
Herkenhoff, K. E.
Hurowitz, J. A.
King, P. L.
Le Mouélic, S.
Leshin, L. A.
Léveillé, R.
Lewis, K. W.
Mangold, N.
Maurice, S.
Ming, D. W.
Morris, R. V.
Nachon, M.
Newsom, H. E.
Ollila, A. M.
Perrett, G. M.
Rice, M. S.
Schmidt, M. E.
Schwenzer, S. P.
Stack, K.
Stolper, E. M.
Sumner, D. Y.
Treiman, A. H.
VanBommel, S.
Vaniman, D. T.
Vasavada, A.
Wiens, R. C.
Yingst, R. A.
Kemppinen, Osku
Bridges, Nathan
Johnson, Jeffrey R.
Minitti, Michelle
Cremers, David
Farmer, Jack
Godber, Austin
Wadhwa, Meenakshi
Wellington, Danika
McEwan, Ian
Newman, Claire
Richardson, Mark
Charpentier, Antoine
Peret, Laurent
Blank, Jennifer
Weigle, Gerald
Li, Shuai
Milliken, Ralph
Robertson, Kevin
Sun, Vivian
Baker, Michael
Edwards, Christopher
Farley, Kenneth
Griffes, Jennifer
Miller, Hayden
Newcombe, Megan
Pilorget, Cedric
Siebach, Kirsten
Brunet, Claude
Hipkin, Victoria
Marchand, Geneviève
Sánchez, Pablo Sobrón
Favot, Laurent
Cody, George
Steele, Andrew
Flückiger, Lorenzo
Lees, David
Nefian, Ara
Martin, Mildred
Gailhanou, Marc
Westall, Frances
Israël, Guy
Agard, Christophe
Baroukh, Julien
Donny, Christophe
Gaboriaud, Alain
Guillemot, Philippe
Lafaille, Vivian
Lorigny, Eric
Paillet, Alexis
Pérez, René
Saccoccio, Muriel
Yana, Charles
Armiens‐Aparicio, Carlos
Rodríguez, Javier Caride
Blázquez, Isaías Carrasco
Gómez, Felipe Gómez
Gómez-Elvira, Javier
Hettrich, Sebastian
Malvitte, Alain Lepinette
Jiménez, Mercedes Marín
Martínez-Frías, Jesús
Martín-Soler, Javier
Martín-Torres, F. Javier
Jurado, Antonio Molina
Mora-Sotomayor, Luis
Caro, Guillermo Muñoz
López, Sara Navarro
Peinado-González, Verónica
Pla-García, Jorge
Manfredi, José Antonio Rodriguez
Romeral-Planelló, Julio José
Fuentes, Sara Alejandra Sans
Martinez, Eduardo Sebastian
Redondo, Josefina Torres
Urqui-O'Callaghan, Roser
Mier, María-Paz Zorzano
Chipera, Steve
Lacour, Jean-Luc
Mauchien, Patrick
Sirven, Jean-Baptiste
Manning, Heidi
Fairén, Alberto
Hayes, Alexander
Joseph, Jonathan
Squyres, Steven
Sullivan, Robert
Thomas, Peter
Dupont, Audrey
Lundberg, Angela
Melikechi, Noureddine
Mezzacappa, Alissa
DeMarines, Julia
Grinspoon, David
Reitz, Günther
Prats, Benito
Atlaskin, Evgeny
Genzer, Maria
Harri, Ari-Matti
Haukka, Harri
Kahanpää, Henrik
Kauhanen, Janne
Kemppinen, Osku
Paton, Mark
Polkko, Jouni
Schmidt, Walter
Siili, Tero
Wray, James
Wilhelm, Mary Beth
Poitrasson, Franck
Patel, Kiran
Gorevan, Stephen
Indyk, Stephen
Paulsen, Gale
Bish, David
Schieber, Juergen
Gondet, Brigitte
Langevin, Yves
Geffroy, Claude
Baratoux, David
Berger, Gilles
Cros, Alain
d’Uston, Claude
Lasue, Jérémie
Lee, Qiu-Mei
Meslin, Pierre-Yves
Pallier, Etienne
Parot, Yann
Pinet, Patrick
Schröder, Susanne
Toplis, Mike
Lewin, Éric
Brunner, Will
Heydari, Ezat
Achilles, Cherie
Oehler, Dorothy
Sutter, Brad
Cabane, Michel
Coscia, David
Israël, Guy
Szopa, Cyril
Robert, François
Sautter, Violaine
Buch, Arnaud
Stalport, Fabien
Coll, Patrice
François, Pascaline
Raulin, François
Teinturier, Samuel
Cameron, James
DeLapp, Dorothea
Dingler, Robert
Jackson, Ryan Steele
Johnstone, Stephen
Lanza, Nina
Little, Cynthia
Nelson, Tony
Williams, Richard B.
Jones, Andrea
Kirkland, Laurel
Baker, Burt
Cantor, Bruce
Caplinger, Michael
Davis, Scott
Duston, Brian
Edgett, Kenneth
Fay, Donald
Hardgrove, Craig
Harker, David
Herrera, Paul
Jensen, Elsa
Kennedy, Megan R.
Krezoski, Gillian
Krysak, Daniel
Lipkaman, Leslie
Malin, Michael
McCartney, Elaina
McNair, Sean
Nixon, Brian
Posiolova, Liliya
Ravine, Michael
Salamon, Andrew
Saper, Lee
Stoiber, Kevin
Supulver, Kimberley
Van Beek, Jason
Van Beek, Tessa
Zimdar, Robert
French, Katherine Louise
Iagnemma, Karl
Miller, Kristen
Summons, Roger
Goesmann, Fred
Goetz, Walter
Hviid, Stubbe
Johnson, Micah
Lefavor, Matthew
Lyness, Eric
Breves, Elly
Fassett, Caleb
Blake, David F.
Bristow, Thomas
Edwards, Laurence
Haberle, Robert
Hoehler, Tori
Hollingsworth, Jeff
Kahre, Melinda
Keely, Leslie
McKay, Christopher
Wilhelm, Mary Beth
Bleacher, Lora
Brinckerhoff, William
Choi, David
Dworkin, Jason P.
Eigenbrode, Jennifer
Floyd, Melissa
Freissinet, Caroline
Garvin, James
Glavin, Daniel
Harpold, Daniel
Jones, Andrea
Mahaffy, Paul
Martin, David K.
McAdam, Amy
Pavlov, Alexander
Raaen, Eric
Smith, Michael D.
Stern, Jennifer
Tan, Florence
Trainer, Melissa
Meyer, Michael
Posner, Arik
Voytek, Mary
Anderson, Robert C.
Aubrey, Andrew
Beegle, Luther W.
Behar, Alberto
Blaney, Diana
Brinza, David
Christensen, Lance
Crisp, Joy A.
DeFlores, Lauren
Ehlmann, Bethany
Feldman, Jason
Feldman, Sabrina
Flesch, Gregory
Jun, Insoo
Keymeulen, Didier
Maki, Justin
Mischna, Michael
Morookian, John Michael
Parker, Timothy
Pavri, Betina
Schoppers, Marcel
Sengstacken, Aaron
Simmonds, John J.
Spanovich, Nicole
Juarez, Manuel de la Torre
Webster, Christopher R.
Yen, Albert
Archer, Paul Douglas
Cucinotta, Francis
Jones, John H.
Niles, Paul
Rampe, Elizabeth
Nolan, Thomas
Fisk, Martin
Radziemski, Leon
Barraclough, Bruce
Bender, Steve
Berman, Daniel
Dobrea, Eldar Noe
Tokar, Robert
Williams, Rebecca M. E.
Cleghorn, Timothy
Huntress, Wesley
Manhès, Gérard
Hudgins, Judy
Olson, Timothy
Stewart, Noel
Sarrazin, Philippe
Vicenzi, Edward
Wilson, Sharon A.
Bullock, Mark
Ehresmann, Bent
Hamilton, Victoria
Hassler, Donald
Peterson, Joseph
Rafkin, Scot
Zeitlin, Cary
Fedosov, Fedor
Golovin, Dmitry
Karpushkina, Natalya
Kozyrev, Alexander
Litvak, Maxim
Malakhov, Alexey
Mitrofanov, Igor
Mokrousov, Maxim
Nikiforov, Sergey
Prokhorov, Vasily
Sanin, Anton
Tretyakov, Vladislav
Varenikov, Alexey
Vostrukhin, Andrey
Kuzmin, Ruslan
Wolff, Michael
Botta, Oliver
Drake, Darrell
Bean, Keri
Lemmon, Mark
Lee, Ella Mae
Sucharski, Robert
Hernández, Miguel Ángel de Pablo
Ávalos, Juan José Blanco
Ramos, Miguel
Kim, Myung-Hee
Malespin, Charles
Plante, Ianik
Muller, Jan-Peter
Navarro-González, Rafael
Ewing, Ryan
Boynton, William
Downs, Robert
Fitzgibbon, Mike
Harshman, Karl
Morrison, Shaunna
Dietrich, William
Kortmann, Onno
Palucis, Marisa
Williams, Amy
Lugmair, Günter
Wilson, Michael A.
Rubin, David
Jakosky, Bruce
Balic-Zunic, Tonci
Frydenvang, Jens
Jensen, Jaqueline Kløvgaard
Kinch, Kjartan
Koefoed, Asmus
Madsen, Morten Bo
Stipp, Susan Louise Svane
Boyd, Nick
Pradler, Irina
Jacob, Samantha
Owen, Tobias
Rowland, Scott
Atlaskin, Evgeny
Savijärvi, Hannu
Boehm, Eckart
Böttcher, Stephan
Burmeister, Sönke
Guo, Jingnan
Köhler, Jan
García, César Martín
Mueller-Mellin, Reinhold
Wimmer-Schweingruber, Robert
McConnochie, Timothy
Benna, Mehdi
Franz, Heather
Bower, Hannah
Brunner, Anna
Blau, Hannah
Boucher, Thomas
Carmosino, Marco
Atreya, Sushil
Elliott, Harvey
Halleaux, Douglas
Rennó, Nilton
Wong, Michael
Pepin, Robert
Elliott, Beverley
Spray, John
Thompson, Lucy
Williams, Joshua
Vasconcelos, Paulo
Bentz, Jennifer
Nealson, Kenneth
Popa, Radu
Kah, Linda C.
Moersch, Jeffrey
Tate, Christopher
Day, Mackenzie
Kocurek, Gary
Hallet, Bernard
Sletten, Ronald
Francis, Raymond
McCullough, Emily
Cloutis, Ed
ten Kate, Inge Loes
Kuzmin, Ruslan
Arvidson, Raymond
Fraeman, Abigail
Scholes, Daniel
Slavney, Susan
Stein, Thomas
Ward, Jennifer
Berger, Jeffrey
and
Moores, John E.
2014.
Elemental Geochemistry of Sedimentary Rocks at Yellowknife Bay, Gale Crater, Mars.
Science,
Vol. 343,
Issue. 6169,
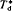 (suspended load/dissolved load from chemical weathering) and Tb/Ts (bed load/suspended load), with any two geochemical components present in the source rock and the alluvial system. If the dissolved load is unknown the ratios can be estimated from the relatively insoluble silica and alumina. The ratio Ts/
(suspended load/dissolved load from chemical weathering) and Tb/Ts (bed load/suspended load), with any two geochemical components present in the source rock and the alluvial system. If the dissolved load is unknown the ratios can be estimated from the relatively insoluble silica and alumina. The ratio Ts/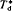 , which for large river basins depends on climate and relief, can thus potentially be determined from ancient alluvial sequences.
, which for large river basins depends on climate and relief, can thus potentially be determined from ancient alluvial sequences.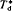 and alluvial geochemistry are known. These rivers together drain 27% of the continental surface. For a source area with the average continental sandstone to shale ratio of 0·6 the observed average value of Ts/
and alluvial geochemistry are known. These rivers together drain 27% of the continental surface. For a source area with the average continental sandstone to shale ratio of 0·6 the observed average value of Ts/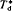 is obtained when limestone, sandstone and shale are present in the proportions 6·7:21·6:35·7. The figure of 64% sediment in the source area is very similar to the 66% determined by Blatt and Jones (1975) from geological maps of the continents. The equations also show that average bed load transport rate into these 13 basins is about 27% of total transport, and into the Amazon basin about 37%. Bed load transport rates out of the basins, into the sea, are relatively very small.
is obtained when limestone, sandstone and shale are present in the proportions 6·7:21·6:35·7. The figure of 64% sediment in the source area is very similar to the 66% determined by Blatt and Jones (1975) from geological maps of the continents. The equations also show that average bed load transport rate into these 13 basins is about 27% of total transport, and into the Amazon basin about 37%. Bed load transport rates out of the basins, into the sea, are relatively very small.

