Introduction
Occupational exposure carries significant risk of threatening the safety of health care workers (HCWs). During the severe acute respiratory syndrome (SARS) outbreak in 2003, 931 HCWs were infected, accounting for 19% of all clinically diagnosed cases 1 ; whereas, during the novel coronavirus disease (COVID-19) pandemic, over 1700 HCWs in Wuhan have been infected and developed COVID-19-related diseases due to various types of occupational exposures. 2 Protecting the safety and well-being of frontline HCWs has drawn growing concerns all over the world. In clinical practice, the occupational exposure events during the pandemic include not only respiratory exposure, but also blood-related and other mucosal exposures. In the hospitals specializing exclusively on COVID-19 medical care in the epidemic regions, occupational exposure has become one of the most critical risks threatening the lives and health of HCWs. 3 Reference Guo, Wang and Zhang4 Based on the information of an unpublished report on occupational exposure from a COVID-19 hospital in Wuhan, the frequency of occupational-related adverse events (ORAEs) in contaminated areas appeared relatively high. Large scale studies on ORAEs in contaminated areas and the risk of occupational exposure for HCWs were rare before the COVID-19 pandemic. This study attempted to identify the prevalence of ORAEs in HCWs working in the contaminated areas, to explore the incidence and risk factors of various adverse events (AEs), and to determine the effectiveness of bundled interventions during the outbreak of emerging severe respiratory infectious diseases, providing evidences to establish reasonable and scientific interventions to decrease the risk of ORAEs.
Methods
Study Subjects
A total of 143 HCWs in a special team urgently assembled for a COVID-19 specialized hospital to work in Wuhan from February 16 to March 26, 2020, were asked to join this investigation by completing a questionnaire. The inclusion criteria are having a clear awareness, the ability to understand the content of the questionnaire, and voluntarily signing an informed consent. Incomplete and obviously inappropriate questionnaires were excluded. A total of 138 (96.5%) valid questionnaires were returned.
The information obtained from the questionnaire included age, gender, specialty and qualification of the HCW, weather temperature (highest and lowest) of the workday, number of times entering as well as the duration in the contaminated area, ORAEs. Self-identified causes of ORAEs were documented by the study participants, including personal protective equipment (PPE)-related factors (eg, mask suffocation, goggle oppression, goggles/eyeglasses fogging, and protective clothing sultriness) and non-PPE-related factors (eg, insomnia, car sickness, dysphoria, dyspepsia, and overfatigue).
The clinical presentations included in the ORAEs are nausea, vomiting, dizziness, headache, tenderness, palpitation, dyspnea, blurred vision, falls, epistaxis, and stick/cut injuries. Tenderness was defined as pain of skin caused by redness, blisters, or even ulcer at the site of facial bony eminence (such as nose, zygoma, forehead, and back of the auricle), due to wearing the mask, goggles, and protective face screen. Occupational exposure events were defined as needle stick/cut exposure, broken skin/mucosal exposure, and respiratory exposure during occupational activities.
Methods
This is a self-controlled study. The participants were given bundled interventions. The differences in the incidence of occupational exposure events and prevalence of ORAEs before (February 16 to February 22, 2020) and after the interventions (February 23 to March 26, 2020) were compared.
The Bundled Interventions
Based on the factors discovered from our previous experience in the infectious disease unit and at the early stage of the special team, each participant received bundled interventions (Tables 1 and 2).
Table 1. Protocols used for the bundled interventions
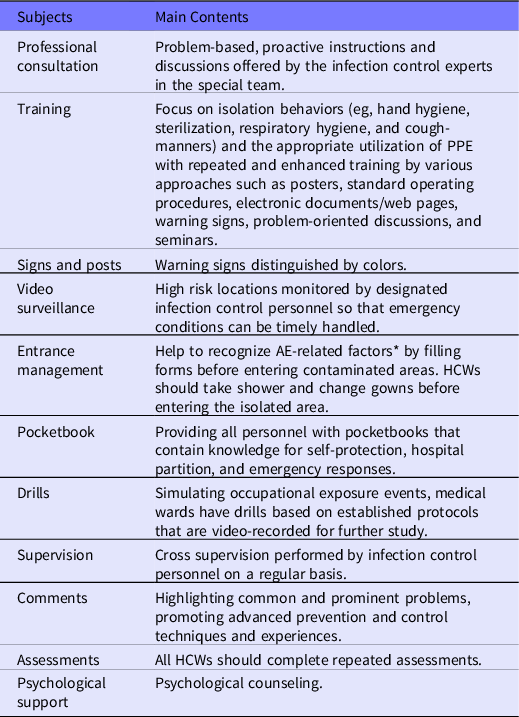
*Details in Table 2.
PPE: personal protective equipment; AE: adverse event; HCWs: health care workers.
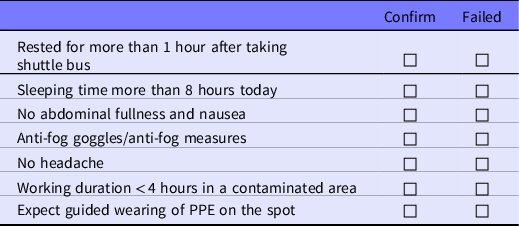
Table 2. Checklist for prevention of ORAEs, to be completed before entering the contaminated areas
Data Processing and Statistical Analysis
All investigations were implemented via Wenjuanxing System, a popular and largest online questionnaire platform in China. Statistical analyses were performed with SPSS statistical software version 25.0 (IBM Corp, Armonk, NY). Quantitative data were shown with $\overline x \pm s$ according to distribution characteristics. Count data were represented by n (%). Two independent samples, t and chi-square tests, were used in group comparisons with quantitative and count data, respectively. A multivariate logistic regression analysis was conducted to analyze the risk factors. P values < 0.05 were considered to be significant.
Results
Demographic Information
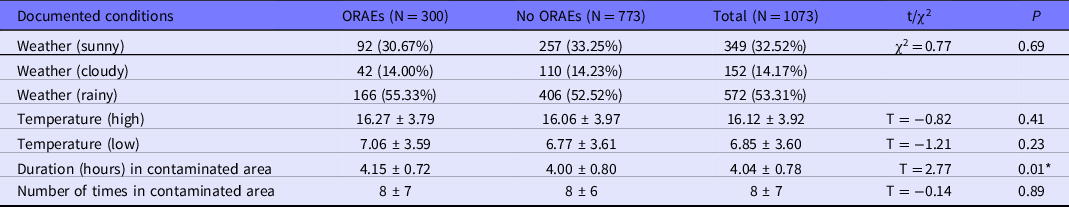
Table 3 shows basic demographic information of the HCWs who participated in this investigation. Ninety one of 138 HCWs (65.94%) had reported at least 1 incidence of ORAEs in the contaminated areas by March 26, 2020. Within 1073 entries into a contaminated area, 300 (27.96%) incidents of ORAEs were recorded. Table 4 shows the conditions of each person entering into contaminated areas. There are no significant differences among groups in terms of age, gender, occupation, qualification, weather, and temperature range (highest and lowest) conditions while entering contaminated areas, and the number of times worked in the contaminated areas. However, a longer duration of working in contaminated areas (4.15 ± 0.72) is significantly associated with ORAEs versus the duration (4.00 ± 0.80) of no ORAEs group, indicating that the length of working in contaminated areas may be a risk factor of developing ORAEs.
Table 3. Demographics of study participants
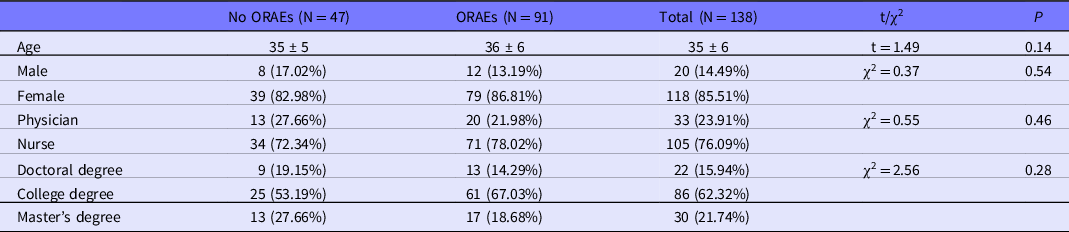
Table 4. Conditions of HCWs entering contaminated area
Risk Factor Analysis
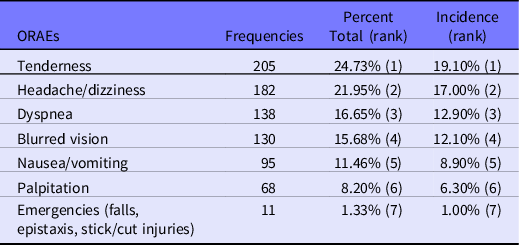
The incidence and rank of ORAEs documented in contaminated areas are shown in Table 5. Tenderness (205 times, 24.73% of all ORAEs), headache/dizziness (182 times, 21.95%), dyspnea (138 times, 16.65%), blurred vision (130 times, 15.68%), and nausea/vomiting (95 times, 11.46%) were the top 5 ORAEs documented.
Table 5. Frequencies, percent of total ORAEs, and incidences of various ORAEs in contaminated area
Relationships between ORAEs and presumed risk factors are shown in Figure 1. All ORAEs were significantly related to the use of PPE. Insomnia was associated with most ORAEs except blurred vision, although we are not certain whether it is the cause or the result of ORAEs, or both. Further detailed results are shown in Figure 2A to 2F.
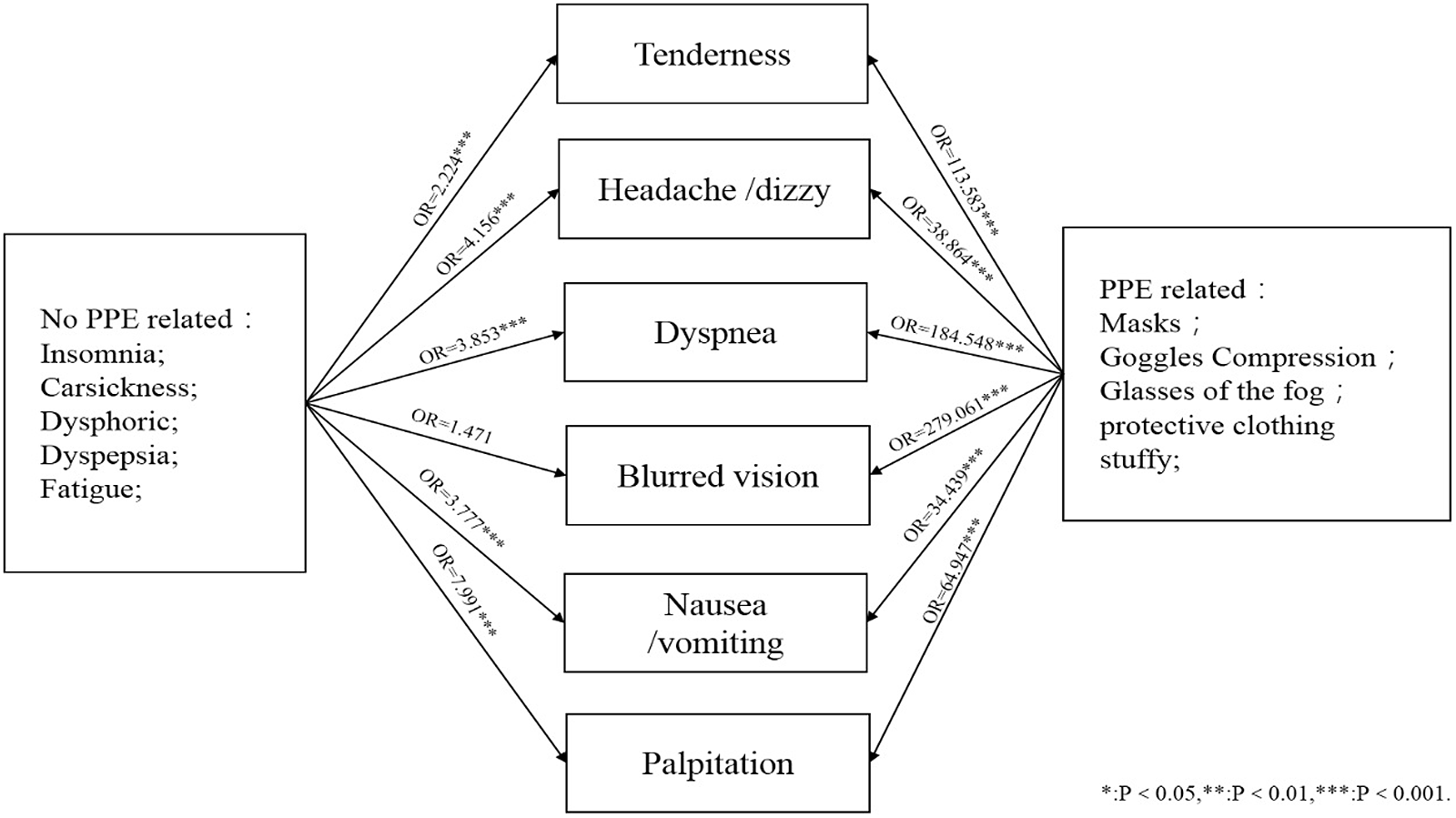
Figure 1. Relationship between ORAEs and related factors.
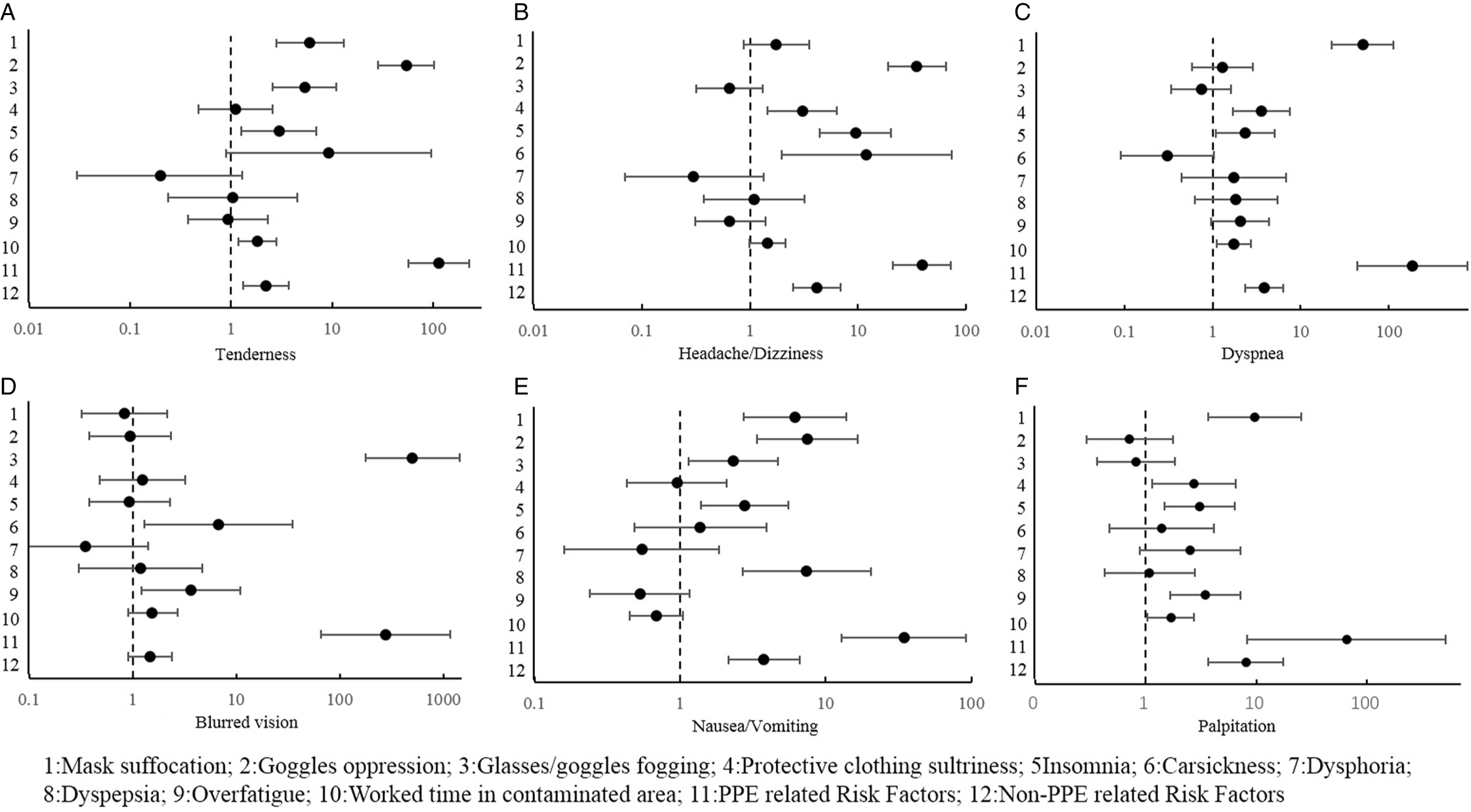
Figure 2. Logistic analysis of ORAEs and risk factors.
Tenderness was mainly correlated with PPE-related factors, such as mask suffocation, goggles oppression, and glasses/goggles fogging. Work time in the contaminated area may be an independent risk factor for tenderness. For every 1 hour increased working in the contaminated area, the risk of tenderness would increase by 81.8%. Meanwhile, tenderness may be associated with insomnia, as shown in Figure 2A.
Headache and dizziness were correlated with goggles oppression, the sultriness of protective suit, insomnia, and car sickness, as shown in Figure 2B.
Dyspnea was correlated with mask suffocation, sultriness of protective suit, insomnia, and work time in the contaminated areas, as shown in Figure 2C.
Blurred vision was correlated with glasses/goggles fogging, overfatigue, and car sickness. In fact, overfatigue and car sickness may increase the risk of blurred vision in the contaminated areas, as shown in Figure 2D.
Mask suffocation, goggles oppression, and glasses/goggles fogging may also be correlated with nausea/vomiting in the contaminated areas, although the main reason was recent dyspepsia. Insomnia may also increase the risk of nausea/vomiting, as shown in Figure 2E.
Insomnia, overfatigue, mask suffocation, and protective suit sultriness may increase the risk of palpitation. For each hour increase in work time in a contaminated area, the likelihood of palpitation would increase by nearly 68.3%, as shown in Figure 2F.
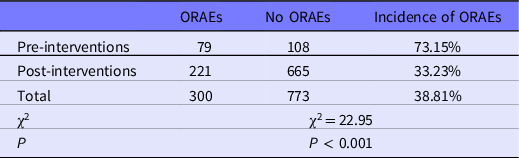
The comparison of occupational exposure events and ORAEs pre- and post-interventions is shown in Tables 6 and 7. The incidence of occupational exposure events and the times of ORAEs in contaminated areas were both different pre- and post-interventions. The incidence of occupational exposure events before interventions was 0.4%, which was higher than post-intervention (0.05%); the difference was statistically significant (χ2=61.49, P < 0.001). The incidence density of ORAEs in contaminated areas before interventions was 73.15%, which was significantly higher (χ2 = 22.95, P < 0.001) than post-intervention (33.23%).
Table 6. Incidence of occupational exposure events before and after intervention
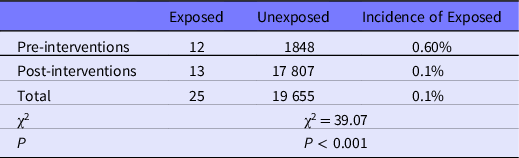
Table 7. Prevalence of ORAEs in contaminated area before and after interventions
Discussion
The COVID-19 pandemic highlighted the necessity of health agencies worldwide to be well prepared to combat large-scale emerging infectious diseases. In the context of the outbreak of a novel severe respiratory infectious disease, especially at the early stage, reliable information of the pathogenic etiology and mechanism, mode of transmission, clinical presentation characteristics, and susceptible populations is lacking – and the availability of effective vaccines can be months to years away. To prevent cross-infection in hospitals, which may represent major spreading events of the infection and a significant impact to the occupational safety of HCWs, a standard and effective protocol of occupational protection is essential. Protective clothing, masks, and other PPE for HCWs entering wards to manage patients are already well accepted in the infectious disease units. In China, the dates when the peak of COVID-19 occurred were particularly unusual. It was during the traditional Spring Festival when manufacturing work was suspended almost all over China; thus, there was a marked shortage of production and supply of the PPEs. The vital role of medical care in the event of a huge number of patients, the fear of the unknown due to lack of sufficient information on the disease itself, and the pressure of public opinion place HCWs under huge psychological pressure. We experienced a stage when ORAEs, such as nausea/vomiting, headache/dizziness, tenderness, palpitation, dyspnea, and blurred vision, frequently occur in contaminated areas, and the incidence of occupational exposure, such as respiratory exposure, syncope, and needle stick injuries, was also relatively high, causing great physical and psychological burdens on HCWs. Reference Tang, Lu and Cai5
This study found that a long duration use of PPEs was the most important cause of ORAEs, which might be related to generally high-level protection while the effectiveness and necessity were not clearly defined. Although it is clearly not advisable to lower the protection level when the transmission mode of a novel severe respiratory infectious disease is unclear, optimizing the procedures of PPEs should be one of the key research areas to improve occupational safety for HCWs and enhance the quality of medical care, so that we can be more prepared for the emerging infectious diseases in the future. There are several key components of PPEs, and each may be improved to alleviate the risk of ORAEs. The protective clothing usually becomes sultry easily. Improving the air permeability of protective clothing material could be a practical way to reduce the burden of long-duration work while wearing these gowns. In practice, Tyvek medical protective clothing has excellent air permeability, with effective protectiveness, and has been highly approved for frontline HCWs. Reference Lu and Zhang6 Mask suffocation is a major factor of ORAES. We think the following measures can be considered to reduce AEs: First, reducing the respiratory resistance by better manufacturing; second, decreasing the work duration and intensity by increasing the number of HCWs per shift would certainly improve the subjective feeling of mask suffocation; third, as irritating smells of masks can aggregate the perception of ORAEs, increasing the purity of the materials and improving the manufacturing process may help reduce some uncomfortable smells of masks. Many studies have reported that pressure injuries on skin associated with medical devices are caused by tightly worn goggles and masks. These injuries not only cause skin damage and pain, but also increase the risk of skin infection. We found that the use of protective dressing can reduce local friction, help absorb exudation, and promote repair while still ensuring that the airtightness of the mask and goggles are adjusted properly. If any pressure injuries are found, they should be properly treated. Antifogging agents applied to goggles are the most common way to prevent blurred vision. Although many goggles and protective face screens have antifogging features, moisture-resistant coatings are frequently destroyed by disinfectants. Povidone-iodine or detergents, such as handwashing solutions, may be reasonable substitutes when antifogging agents are in shortage. Anionic surfactants in detergents can reduce surface tension effectively to achieve moisture resistance; molecular iodine formed after povidone-iodine oxidation may also achieve moisture protection. Those alternative methods can generally achieve more than 2-hour antifogging effects, reducing the occurrence of ORAEs caused by blurred vision. Reference Li and Cao7 Last, but not least, overuse and misuse of PPE should also be discouraged. Overuse of PPE by overlapping/double protective clothes, goggles, and masks results in a significant waste of already limited medical resources and increased disposal burden. Among non-PPE-related factors, HCWs were found to have a higher prevalence of insomnia, and it was also associated with most ORAEs in contaminated areas. Similar results have been reported in previous studies. Reference Nicolle8–10 When providing diagnoses and treatments to critically ill patients, due to frequent overtime work with higher intensity and occupational safety risk, HCWs would inevitably break sleep habits, which then lead to disruption of the biological clock. Long-term and persistent stressful events not only increase the risk of newly onset insomnia, but also enhance chronic insomnia. To mitigate the burden of insomnia for this group of people, the prevailing protocol is to deploy professional psychotherapists with active individual intervention, supplemented by the use of sedative hypnotic medicines. On the other hand, Car sickness is a risk factor that arises in a particular background. During the COVID-19 outbreak in Wuhan, HCWs were gathered in dormitories located more than 40 kilometers from the hospital and commuted with specially assigned shuttle buses so as to facilitate workforce management and to avoid cross-infection. As a result, multiple cases of ORAEs in contaminated areas associated with car sickness had been observed. Therefore, measures to reduce car sickness, such as reducing commute time by moving to closer lodging, training drivers to avoid operations that might induce car sickness, and distributing prophylactic pills to HCWs vulnerable to car sickness, may decrease the risk of ORAEs. Additionally, working duration in the contaminated “red zone” was also a crucial risk factor. The increased risk was associated not only with the clinical workload, but also the lengthy procedure to take off PPEs. There are potential risks of infection with several HCWS undressing PPE simultaneously within a limited space. Actually, there had been reports of infection events due to inappropriate PPE undressing every now and then. 11 , 12 Therefore, HCWs had to undress PPEs sequentially following a strict protocol, which became a factor contributing to extended work time in contaminated areas. The problem became serious when numerous HCWs were required to enter the contaminated area at the same time as a large number of patients were admitted. Additional ameliorating measures to reduce risk of ORAEs for non-PPE related factors include setting up reasonable shift arrangement and increasing the number of HCWs per shift to alleviate fatigue and insomnia. In our experience, screening of AEs (eg, car sickness, insomnia) before entering contaminated areas, and appropriate intervention, when necessary, helped effectively reduce the negative impact of non-PPE-related factors. Needless to say, sufficient instructions and training on PPE on/off protocols are critical to ensure that HCWs are cultivating a good routine habit. One of the main reasons for occupational exposure at the early stage of the COVID-19 outbreak was insufficient protection for HCWs due to lack of PPE stocks and poorly implemented infection control measures. A study has confirmed that sufficient PPE could effectively decrease the occupational exposure of HCWs. Reference Chen, Wei and Wang13 Even if given sufficient supplies and assuming all being used adequately and reasonably, it’s still extremely important to ensure a thorough implementation of infection control measures, especially when many HCWs without much experience in dealing with infectious disease join the special workforce to combat the pandemic. For instance, by conveying the knowledge, improving the perception and behavior, strengthening the notion that “behavioral isolation” is superior to “physical isolation,” it is effective to maximize the contribution of PPEs in our experience.
One advantage of our bundled intervention model is the ability to make all aspects of the infection and AE risk controllable. Professional measures of infection control can not only decrease the infection rate, Reference Correa-Martínez, Schwierzeck and Mellmann14 but also increase HCWs’ confidence to work against the viral infection. At the early stage of the pandemic, concerns and fears related to COVID-19 were prevalent among HCWs. Reference Shreffler, Petrey and Huecker15 So far, a majority of interventional research studies have assessed occupational exposure based on the SHEL model Reference Guo, Lu and Wang16,Reference Molloy and O’Boyle17 and the Reason model Reference Reason18 ; these methods analyzed occupational exposure caused by mismatchs between individual factors and the corresponding microenvironment from the perspective of system theory. The bundled interventions of this study were based on the findings of early investigations and then focused on taking actions against the most relevant issues. The bundled interventions had been effective, as indicated by the results of this study. Through these interventions, many HCWs became more compliant with the protection procedures; some incorrect perceptions, such as the notion that more and thicker PPEs offer better protection, were scientifically addressed. The results of our investigation and implementation in clinical practice indicate that this intervention model has high effectiveness and feasibility.
Limitations
The limitations of this study are mainly reflected in 2 aspects. First, the self-control at different time points of clinical practice in this study is imperfect, as the HCWs are likely to get more experienced to deal with the daily routine at the later stage of this study. However, it is difficult to set a control to eliminate the influence of experience on our study results. Second, the statistical analyses of various types of ORAEs are limited by sample size. Thus, more intensive and in-depth studies are warranted in the future to support our results and conclusions.
Conclusion
In summary, our study indicates that HCWs have suffered a high risk of occupational exposure and a high prevalence of ORAEs working in contaminated areas, related to various factors during the early stage of the COVID-19 epidemic. Timely, comprehensive, and multi-level interventions can significantly decrease the incidence of ORAEs and occupational exposures for HCWs working in contaminated areas.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/dmp.2021.237
Author contributions
Co-first authors TL and YG have contributed equally to this study.
Funding statement
This study is supported by the annual scientific research project of Taikang Tongji Hospital (TKTJKY2020154, TKTJKY2020155); technological innovation in major field of military medicine of the Southwest Hospital (SWH2017ZDCX2005).
Conflict(s) of interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this paper.











