Mpox, a member of the Orthopoxvirus family (formerly known as monkeypox), is a re-emerging zoonotic infection. It was first observed in a colony of cynomolgus monkeys (Macaca fascicularis) in 1958 in Copenhagen, Denmark.Reference Bonilla-Aldana and Rodriguez-Morales1 The first human cases were identified later in 1970, in the Democratic Republic of Congo, where sporadic human-to-human transmission was described in some Western and Central African countries, mainly among children in rural, rainforest areas.Reference Mitjà, Ogoina and Titanji2 Due to the increasingly widespread outbreaks of Mpox being reported globally, the World Health Organization (WHO) declared Mpox a Public Health Emergency of International Concern (PHEIC) on July 23, 2022.3 As of March 5, 2024 the US Center for Disease Control and Prevention (CDC) reported Mpox outbreaks in 118 countries, with the total number of polymerase chain reaction (PCR)-confirmed Mpox cases as 94.274 cases.4
A study in the Democratic Republic of Congo from 2000-2015 reported that the emergence of human Mpox cases is associated with outbreaks of Mpox in nearby animal populations.Reference Mandja, Brembilla and Handschumacher5 More recently, studies elsewhere have reported that the spread outside of Africa has been related to the importation of infected animals into non-endemic countries, and human travel to endemic countries.Reference Erez, Achdout and Milrot6 Non-travel-related (autochthonous) Mpox cases have also been reported from Germany.Reference Kupferschmidt7 Studies in several countries have since identified that Mpox transmission occurred through sexual or other intimate contact among men who have sex with men.Reference Kupferschmidt7, Reference Allan-Blitz and Klausner8 Due to the varied mode of transmission, including direct skin-to-skin and body fluids contact, respiratory aerosols, sexual intercourse, and contact with contaminated objects,Reference Thornhill, Barkati and Walmsley9 it has become necessary to develop effective infection prevention and control measures in health care and community settings.
The Republic of Indonesia has been reported as one of the biggest territories with a favorable Mpox sustainable environment, with one of the largest populations at risk of infection.Reference Sun, Chen and Liu10 The first case of Mpox in Indonesia emerged in August 2022. Subsequently, Mpox cases were reported again in October 2023 and have increased in number through November 2023,11 with an update on December 5, 2023 reporting 45 PCR-confirmed Mpox cases.12 Of note, there have been no studies describing the demographic and clinical characteristics for Mpox in Indonesia. A study in 2020 on Mpox from Indonesia found that inadequate knowledge of Mpox among general practitioners could affect missed cases and delayed reporting.Reference Harapan, Setiawan and Yufika13 Also, as the capital city of Indonesia, Jakarta is connected nationally and globally, posing a higher risk of cross-border transmission for any emerging infectious disease. Thus, understanding the demographic and clinical characteristics of Mpox cases is crucial to providing valuable insight for strengthening outbreak preparedness and response, collaborative disease surveillance, enhancing clinical care, infection control measures, and public health mitigation action.Reference Rahi, Joy and Sharma14, 15 To support the public health response efforts to interrupt Mpox transmission in the community, this study aims to describe the epidemiology and clinical features of Mpox in Jakarta, Indonesia.
Materials and Methods
Study Setting
This study utilizes public health surveillance data collected in Jakarta, Indonesia, covering the period from October 2023 to February 2024. Jakarta, the capital of Indonesia, stands as a diverse metropolitan city, serving as the nation’s governmental and economic nucleus, and facilitating both national and international air and sea transportation. The city, with a population of 10 million encompassing Jakarta and its metropolitan area, covers an area of 4384 square kilometers, with a population density of approximately 13 000 people per square kilometer.16 This considerable population density (Supplementary Figure 1) creates a high demand for land in the city, mirroring similar situations found in other megacities around the world.Reference Martinez and Masron17 Following the first PCR-confirmed Mpox cases in August 2022 and October 2023, a total of 58 cases were reported by February 2024.
Indonesian Mpox Surveillance System
The Mpox surveillance system was developed based on the system used for COVID-19.11 Daily reports of Mpox cases were sent from public health centers and hospitals to the Ministry of Health, Indonesia. The method for detecting Mpox, based on WHO guidelines, relies on the detection of Mpox DNA. This is usually achieved using PCR in either real-time or conventional formats, on skin swabs of vesicular lesions or crusts of lesions. For surveillance purposes, this was performed by laboratories in The Provincial of Jakarta and Ministry of Health, Indonesia. Oropharyngeal swabs, anal/rectal swabs, or serum samples, are acceptable alternative test samples.11
A suspected case is defined as a person with a recent onset of rash lesions on the skin or mucous membranes, possibly accompanied by symptoms such as fever, chills, myalgias, headaches, lymphadenopathy, low back pain, asthenia, proctitis, or a history of contact with a confirmed case within the last 21 days, alongside travel history to endemic areas.11 The rash period is the duration from the appearance of lesions to desquamation (14-28 days).Reference Farahat, Sah and El-Sakka18 In cases of Mpox, the rash typically emerges 1 to 5 days after initial symptoms, subsequently evolving into fluid-filled lesions, progressing into pustules, and ultimately culminating in scab formation.Reference Farahat, Sah and El-Sakka18, Reference Ortiz-Martínez, Rodríguez-Morales and Franco-Paredes19 Mpox cases are categorized within 2 groups based on laboratory assessment with PCR testing.11, 20 Suspected or probable Mpox cases with negative PCR tests are defined as discarded cases, while confirmed cases are defined as individuals with a positive PCR test for the Mpox virus.11, 20
In addition, “close contact” is defined as those with a history of contact with suspected or confirmed Mpox cases (from the onset of symptoms until the crust peels off/disappears), who meet one of the following criteria: direct skin-to-skin physical contact (for example, touching, hugging, kissing, and intimate or sexual contact), contact with contaminated objects (fomites) such as clothing or bed linens during the washing or cleaning process of the room, prolonged face-to-face respiratory exposure at close range, respiratory exposure (potential inhalation), or exposure of the eye mucosa to lesions (for example, crusts) from an infected person and exposed health care workers without the use of appropriate personal protective equipment (PPE).11
Data Analysis
This study utilized counts and proportions to delineate the demographic characteristics of confirmed and discarded Mpox cases. The χ Reference Mitjà, Ogoina and Titanji2 test was used to compare the characteristics of confirmed and discarded Mpox cases. Statistical analysis used the R statistical programming software version 4.3.1 (R Foundation for Statistical Computing, Vienna, Austria). An epidemic curve was employed to illustrate the trend of Mpox from epidemiological week 41 (October 8-14, 2023) to week 8 (February 18-24, 2024). Ethical clearance was obtained from the School of Medicine, Universitas Islam Sumatera Utara, Medan, Indonesia (ref 463/EC/KEPK.UISU/XI/2023).
Results
The Mpox surveillance recorded a total of 258 cases, including suspected, confirmed (PCR-positive), and discarded (PCR-negative) cases during the period of October 2023 and February 2024. Of these, the following cases were excluded: 1 suspected case, 8 confirmed and 24 discarded Mpox cases residing outside the study location (such as Depok, Tangerang, and Bekasi), and 12 discarded Mpox cases with missing data. The remaining 213 Mpox cases (including 58 confirmed and 155 discarded) were used in this analysis, incorporating the characteristic shown in Tables 1–3.
Table 1. Socio-demographic characteristics of confirmed and discarded Mpox cases in Jakarta, Indonesia during study period (N = 213)

In the 2023, first confirmed Mpox cases reported in epidemiological week 41 and increased in the consecutive week (Figure 1). Among the confirmed Mpox cases, most were males (96.6%, 56/58), of which 67.2% (39/58) identified themselves as men who have sex with men (MSM). 67.2% (39/58) reported recent sexual activity within 21 days prior to the disease onset date, with 64.9% reporting only 1 sexual partner during that period. 13.8% (8/58) of confirmed cases had a history of smallpox vaccination, close contact with confirmed cases, or contact with wild or domestic animals within the 21 days prior to disease onset (8.6%, 5/58). In terms of comorbidities, 69.0% (40/58) of cases were individuals living with HIV/AIDS, and 37.9% (22/58) had been diagnosed with sexually transmitted diseases (Table 1).

Figure 1 Epidemic curve of PCR-positive (orange bar) and PCR-negative (grey bar) Mpox cases in Jakarta, Indonesia from October 2023 to February 2024.
Among confirmed Mpox cases, common symptoms included fever (81.1%, 47/58), rash (63.6%, 37/58), and lesions (93.1%, 54/58) (Table 2). Notably, these symptoms occurred at higher frequencies compared to discarded Mpox cases (P value < 0.05). Furthermore, rectal pain, rectal bleeding, and genital ulcers showed associations with confirmed Mpox cases (29.3%, 17/58; 10.3%, 6/58; and 20.7%, 12/58). Reported among confirmed cases were vesicular (46.6%, 27/58) and pustule (50.0%, 29/58) lesions (Table 3). Notably, 58.6% (34/58) of total confirmed Mpox cases exhibited a rash in the facial area. Additionally, rash zones were identified in the genital area (36.2%, 21/58) and the perianal region (18.9%, 11/58).
Table 2. Symptoms of Mpox among confirmed and discarded cases (N = 213)
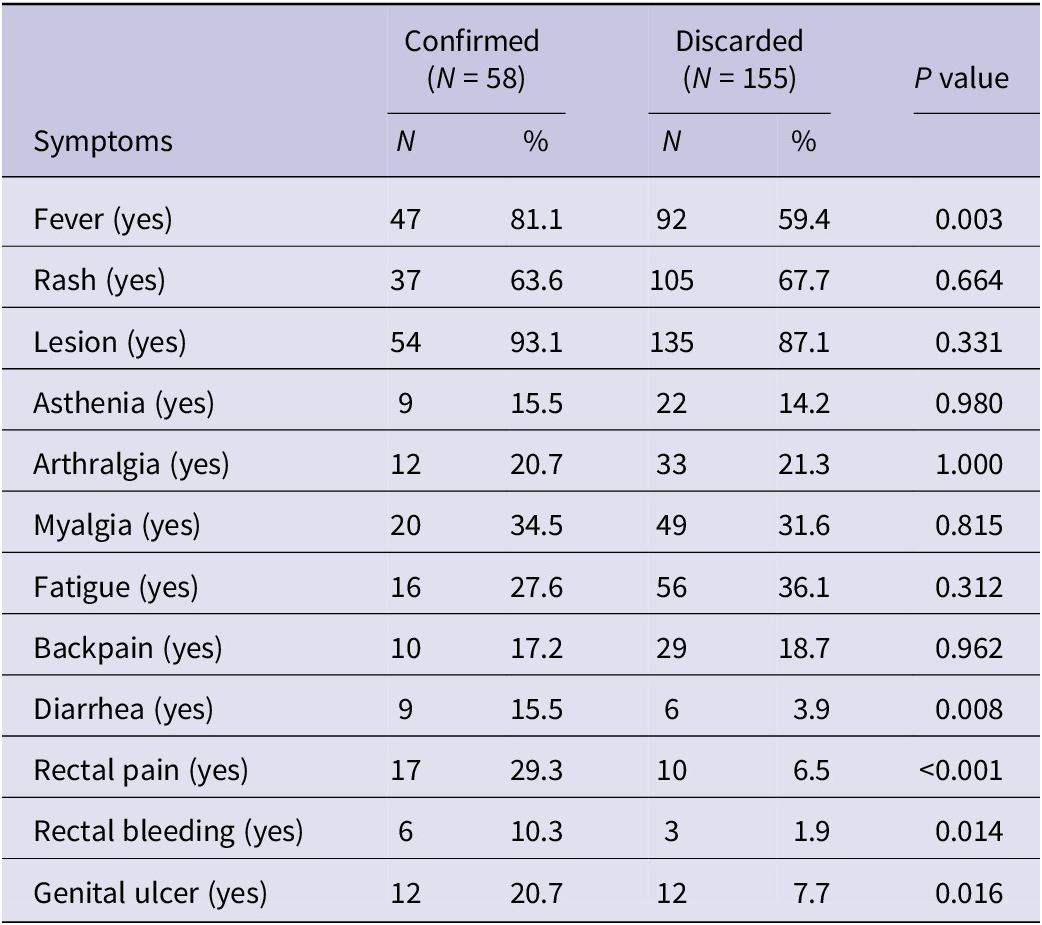
Table 3. Rash zone and lesion type of Mpox among confirmed and discarded cases (N = 213)

Discussion
This study described the Mpox epidemiological surveillance data of confirmed cases (PCR-positive test) in Jakarta, Indonesia. During the period of October 2023 to February 2024, a study identified 58 confirmed and 155 discarded cases (PCR-negative test) of Mpox, with the peak occurring in epidemiological week 44 (from October 30, 2023 to November 5, 2023). Most (96.6%, 56/58) confirmed cases were males, predominantly aged 20-40, with a high proportion of MSMs and recent sexual activity. Other studies report similar findings of male predominance in Mpox cases.Reference Reynolds, Yorita and Kuehnert21, Reference Sharma, Prasad and Kaeley22 The higher prevalence of male cases indicates transmission within sexual networks, specifically among MSMs, rather than being solely attributed to inherent gender vulnerability. According to the WHO, Mpox cases in non-endemic countries have mainly been reported amongst MSMs implicated Mpox transmission.20 Sexual networks among MSM are similar to those of other groups, but a core group is more densely connected, with frequent partner changes and multiple concurrent partners, reducing the likelihood of the virus encountering transmission barriers.Reference Kupferschmidt7 However, this finding probably underestimates of the number of Mpox cases due to fear of stigma and discrimination in the study setting that might have potentially exacerbated this underestimation, and introduced reporting biases in the surveillance reporting system.Reference Laurenson-Schafer, Sklenovská and Hoxha23 Therefore, necessary measures for Mpox elimination and control are essential to ensure optimal health care, integrated sexual health, HIV prevention and care services, and to conduct risk communication aimed at enhancing the ability to mitigate individual risk while reducing stigma and fear.
In the light of the possibility of ascertainment bias, this data highlights the higher percentages of Mpox transmission through sexual intercourse (amongst MSMs, 67.2%, 39/58), especially in those who have had sex within the last 21 days. Additionally, this study indicates that individuals with HIV and syphilis may be affected by shared sexual behaviors and networks that increase the possibility of contracting multiple sexually transmitted infections. Mpox transmission through sexual contactReference Bragazzi, Kong and Mahroum24 is more predominant compared to a history of traveling to Mpox-endemic areas.Reference Angelo, Petersen and Hamer25 This is attributed to the increasing number of non-endemic countries affected by Mpox4 and other research findings indicating its spread through sexual contact.Reference Sharma, Prasad and Kaeley22 The pattern of Mpox cases tends to not lead to outbreaks, but follows a sporadic transmission pattern linked to sexual activity, as evidenced by the epidemic curve pattern in Jakarta, where there is a tendency for a lack of exponential case increases, and sustained increases over a long period. Although this study demonstrates the possibility of transmission through sexual contact, this seems to be confined to MSM networks, rather than heterosexual activity, as there was no reported transmission through heterosexual commercial sex workers during the study period, suggesting that transmission was confined to the core group during this timeframe. A study in California, USA reported that changes in epidemic curves and demographic characteristics in core and noncore areas can help determine where an epidemic has originated or might arise, and identify the most affected groups, thereby guiding the formulation of targeted intervention strategies for controlling the outbreak.Reference Gesink, Sullivan and Miller26
This study found that rashes and lesions were present in both PCR-confirmed and PCR-negative cases. Due to data limitations, the diagnosis in the PCR-negative group could not be reported in this study. However, the rash that appeared could originate from other diseases with rash symptoms, whereas all individuals displaying rash symptoms were considered suspected cases of Mpox during the study period. Surveillance data from the Jakarta provincial health office, accessible to the public, indicates that from September to November 2023, there were approximately 300 cases of dengue hemorrhagic fever, 945 cases of Dengue fever, and 387 reported cases of measles.27 A systematic report found that there is a potential for symptoms to overlap amongst patients diagnosed with Dengue and measles, as well as those with Mpox.Reference Hamdana, Mohsin and Habib Tharwani28 Therefore, it is crucial to strengthen laboratory capacity to establish accurate diagnoses of Mpox in areas with endemic levels of Dengue and measles cases.
This study has several limitations. The routine Mpox surveillance was mostly passive and reported by large public health centers or hospitals, rather than smaller primary or community care centers. This meant that some key baseline variables were either incomplete or unavailable. Comorbidities were often self-reported without confirmation, and could be under-, over-, or mis-diagnosed, potentially affecting the accuracy of any association with Mpox. However, this is the first epidemiological study of Mpox cases in Indonesia to present clinical manifestations. Future research will expand upon this to include other areas and populations within Indonesia, with improved data and analysis methods.
Conclusion
Despite the study limitations, this study highlights the fact that Mpox infection in Jakarta, Indonesia has been frequently observed in males, bisexual people, people with recent history of sexual intercourse, and having comorbidities such as HIV/AIDS, and syphilis. In summary, our findings suggests that the observed predominance of male cases likely indicates transmission within sexual networks, such as among men who have sex with men, rather than an inherent vulnerability associated with gender alone. The higher prevalence of Mpox among individuals with HIV or syphilis is likely attributable to shared sexual behaviors and networks that elevate the risk of multiple sexually transmitted infections, rather than these diseases inherently increasing susceptibility to Mpox. Effective strategies against Mpox infection are needed to address public health challenges on outbreak preparedness and response, including contact tracing and health care services, all of which are essential to reduce the spread of Mpox in the community.
Supplementary material
The supplementary material for this article can be found at http://doi.org/10.1017/dmp.2024.329.






