Introduction
Birth size may be considered a measure of the overall success of fetal development (Schlotz & Phillips, Reference Schlotz and Phillips2009). Very small size at birth reflects impaired fetal growth, premature delivery, or a combination of both. Infants born at extremely low birth weight (ELBW; <1000 g) are among the smallest and most vulnerable who survive. These high-risk neonates are highly prone to postnatal growth failure—growth velocity that is slower than expected—in the first weeks following birth (Cooke et al., Reference Cooke, Ainsworth and Fenton2004; Hsiao et al., Reference Hsiao, Tsai, Chen and Lin2014; Mathews et al., Reference Mathews, Miniño, Osterman, Strobino and Guyer2011). Preterm infants who are more immature (Pridham et al., Reference Pridham, Brown, Sondel, Clark and Green2001) or have significant illness (Cooke, Reference Cooke2010; Ramel et al., Reference Ramel, Brown and Georgieff2014) are especially vulnerable. In most cases, growth failure is not overcome by the time these infants are discharged from hospital (e.g., Ehrenkranz et al., Reference Ehrenkranz, Younes, Lemons, Fanaroff, Donovan, Wright, Katsikiotis, Tyson, Oh, Shankaran, Bauer, Korones, Stoll, Stevenson and Papile1999; Horbar et al., Reference Horbar, Ehrenkranz, Badger, Edwards, Morrow, Soll, Buzas, Bertino, Gagliardi and Bellù2015), despite the provision of high-nutrition intravenous feedings designed to counter cumulative protein and energy deficits accrued in the first weeks of life (Embleton et al., Reference Embleton, Pang and Cooke2001). Postnatal growth failure has been associated with poorer neurodevelopmental outcomes and smaller body size in early childhood (Latal-Hajnal et al., Reference Latal-Hajnal, von Siebenthal, Kovari, Bucher and Largo2003), and poorer neurologic outcomes (Guellec et al., Reference Guellec, Lapillonne, Marret, Picaud, Mitanchez, Charkaluk, Fresson, Arnaud, Flamand, Cambonie, Kaminski, Roze, Ancel, Larroque, Ancel, Blondel, Bréart, Dehan, Garel and Voyer2016) and neurocognitive functioning at school age (Casey et al., Reference Casey, Whiteside-Mansell, Barrett, Bradley and Gargus2006; Ong et al., Reference Ong, Kennedy, Castañeda‐Gutiérrez, Forsyth, Godfrey, Koletzko, Latulippe, Ozanne, Rueda, Schoemaker, Beek, Buuren and Fewtrell2015; see Taine et al., Reference Taine, Charles, Beltrand, Rozé, Léger, Botton and Heude2018, for a recent review).
The earliest growth periods are most significant for long-term development (Mummert et al., Reference Mummert, Schoen, Lampl, Halfon, Forrest, Lerner and Faustman2018). Should adverse conditions prevail during periods of dynamic growth, fetal or infant adaptation to these circumstances may induce programming effects that can permanently alter the structure, function, or set points of the body’s physiological systems (Barker et al., Reference Barker, Osmond, Kajantie and Eriksson2009; Gluckman et al., Reference Gluckman, Hanson, Cooper and Thornburg2008; Metcalfe & Monaghan, Reference Metcalfe and Monaghan2001). Programming effects are most robust during the first 1000 days after conception (Barker, Reference Barker2012). Programming effects initiated during gestation not only lead to changes in physical morphology or physiology, but may also lead to changes in psychological functioning that culminate in internalizing and externalizing behaviors (Schlotz et al., Reference Schlotz, Jones, Godfrey and Phillips2008), increased susceptibility to stress (Cheung et al., Reference Cheung, Khoo, Karlberg and Machin2002; Nilsson et al., Reference Nilsson, Nilsson, Östergren and Rasmussen2004), difficult temperament (e.g., negative affectivity, Pesonen et al., Reference Pesonen, Räikkönen, Kajantie, Heinonen, Strandberg and Järvenpää2006; trait anxiety, Lahti et al., Reference Lahti, Räikkönen, Pesonen, Heinonen, Kajantie, Forsén, Osmond, Barker and Eriksson2010), or mood disorders (Brown et al., Reference Brown, van Os, Driessens, Hoek and Susser2000; de Mola et al., Reference de Mola, De França, de Avila Quevedo and Horta2014; Faa et al., Reference Faa, Manchia, Pintus, Gerosa, Marcialis and Fanos2016). While the notion of prenatal programming has attracted much attention (see Hartmann & Belsky, Reference Hartman, Belsky, Wazana, Székely and Oberlander2021, for a recent review), the question of whether long-term mental health outcomes may be related to early postnatal programming effects has been explored less often.
Mental health risks following extremely preterm birth
Individuals born at ELBW are at greater risk of developing mental health difficulties than their peers born at normal birth weight (NBW; >2500 g; see Johnson & Marlow, Reference Johnson and Marlow2014; Mathewson et al., Reference Mathewson, Chow, Dobson, Pope, Schmidt and Van Lieshout2017; Pyhälä et al., Reference Pyhälä, Wolford, Kautiainen, Andersson, Bartmann, Baumann, Brubakk, Evensen, Hovi, Kajantie, Lahti, Van Lieshout, Saigal, Schmidt, Indredavik, Wolke and Räikkönen2017 for reviews). Problems associated with emotional disorders, (e.g., Johnson et al., Reference Johnson, Hollis, Kochhar, Hennessy, Wolke and Marlow2010; Taylor et al., Reference Taylor, Margevicius, Schluchter, Andreias and Hack2015) and social functioning (e.g., Natalucci et al., Reference Natalucci, Becker, Becher, Bickle, Landolt and Bucher2013; Poole et al., Reference Poole, Saigal, Van Lieshout and Schmidt2020; Woodward et al., Reference Woodward, Moor, Hood, Champion, Foster-Cohen, Inder and Austin2009) are well-documented in children, adolescents, and adults born extremely preterm. Externalizing behaviors are less frequently elevated in preterm children (e.g., Aarnoudse-Moens et al., Reference Aarnoudse-Moens, Weisglas-Kuperus, van Goudoever and Oosterlaan2009; Woodward et al., Reference Woodward, Moor, Hood, Champion, Foster-Cohen, Inder and Austin2009, but see Elgen et al., Reference Elgen, Leversen, Grundt, Hurum, Sundby, Elgen and Markestad2012) and adolescents (Boyle et al., Reference Boyle, Miskovic, Van Lieshout, Duncan, Schmidt, Hoult, Paneth and Saigal2011), except for attention deficits, which are common at both ages (e.g., Anderson et al., Reference Anderson, de Miranda, Albuquerque, Indredavik, Evensen, Van Lieshout, Saigal, Taylor, Raikkonen, Kajantie, Marlow, Johnson, Woodward, Austin, Nosarti, Jaekel, Wolke, Cheong, Burnett and Doyle2021; Lindström et al., Reference Lindström, Lindblad and Hjern2011; Woodward et al., Reference Woodward, Moor, Hood, Champion, Foster-Cohen, Inder and Austin2009). Low birth weight (e.g., Faa et al., Reference Faa, Manchia, Pintus, Gerosa, Marcialis and Fanos2016; Gale & Martyn, Reference Gale and Martyn2004; Nomura et al., Reference Nomura, Brooks-Gunn, Davey, Ham and Fifer2007) and factors limiting fetal growth (e.g., Tore et al., Reference Tore, Antoniou, Reed, Southwood, Smits, McCleery and Zeegers2018) may be implicated in the etiology of affective disorders that emerge later in development.
Unfortunately, the available literature on postnatal growth patterns and mental health problems in preterm populations is not completely consistent across published findings. Cohort studies from the general population suggest that smaller size at birth and in infancy predicts higher levels of trait anxiety in late adulthood (Lahti et al., Reference Lahti, Räikkönen, Pesonen, Heinonen, Kajantie, Forsén, Osmond, Barker and Eriksson2010), but not depressive symptoms (Räikkönen et al., Reference Räikkönen, Pesonen, Kajantie, Heinonen, Forsén, Phillips, Osmond, Barker and Eriksson2007). On the other hand, overall postnatal growth rates in preterm infants may show no relation to mental health problems in adulthood (Sammallahti et al., Reference Sammallahti, Lahti, Pyhälä, Lahti, Pesonen, Heinonen, Hovi, Eriksson, Strang-Karlsson, Järvenpää, Andersson, Kajantie, Räikkönen and Rogers2015). In contrast, faster first-year head growth in preterm infants has been associated with lower risks for childhood depression and ADHD, but not anxiety (Soldateli et al., Reference Soldateli, Silveira, Procianoy, Belfort, Caye, Leffa, Franz, Barros, Santos, Matijasevich, Barros, Tovo-Rodrigues, Menezes, Gonçalves, Wehrmeister and Rohde2022). In adults born late preterm (34–37 weeks’ gestational age), faster head growth from 20 to 56 months has been associated with fewer internalizing, but not externalizing, behaviors (Sammallahti et al., Reference Sammallahti, Heinonen, Andersson, Lahti, Pirkola, Lahti, Pesonen, Lano, Wolke, Eriksson, Kajantie and Raikkonen2017).
Although preterm infants are highly likely to exhibit altered growth trajectories (Embleton et al., Reference Embleton, Pang and Cooke2001), to date, potential effects of postnatal growth patterns on later mental health outcomes remain largely unexplored in individuals born extremely preterm.
The present study
The aim of the current study was to test the hypothesis that altered patterns of postnatal growth in preterm survivors are predictive of internalizing and externalizing behaviors seen during development, using a longitudinal cohort of ELBW survivors followed prospectively from birth. The growth trajectories for weight, height, head circumference (HC), and body mass index (BMI), for this cohort have been described previously (Saigal et al., Reference Saigal, Stoskopf, Streiner, Paneth, Pinelli and Boyle2006). During the first year of postnatal life, these infants were rapidly “losing ground” in weight for age, relative to the 50th percentile for infants of this gestational age (Kuczmarski et al., Reference Kuczmarski, Ogden, Grummer-Strawn, Flegal, Guo, Wei, Mei, Curtin, Roche and Johnson2000). Weight gains in ELBW infants caught up considerably in the second and third years, but final attainment of absolute height, weight, and HC remained lower in ELBW adults than NBW control adults, indicating that the effects of ELBW on adult stature and head size were permanent. These ELBW survivors also displayed higher levels of internalizing behaviors in adolescence (Saigal et al., Reference Saigal, Pinelli, Hoult, Kim and Boyle2003).
In the present study, we tested whether long-standing physiological changes associated with perinatal adversity would predict higher levels of internalizing or externalizing behaviors at later stages of development, consistent with postnatal programming effects (Barker et al., Reference Barker, Osmond, Kajantie and Eriksson2009). It would be logical to examine these problems developmentally, especially in adolescence, a sensitive period often characterized by significant physical and social changes and a rise in mental health problems in general populations (Kessler et al., Reference Kessler, Amminger, Aguilar-Gaxiola, Alonso, Lee and Üstün2007). Therefore, we examined whether growth patterns in early postnatal life, measured by the Ponderal Index (PI) and HC, were associated with internalizing or externalizing behaviors in childhood and adolescence in individuals born at ELBW.
The PI (birth weight × 100 /crown-heel length3) is a proportional measure of the adiposity of newborns (weight per length) that provides a rough estimate of their nutritional status at birth. It has long been used to identify intrauterine growth restriction (IUGR) or soft-tissue “wasting” during fetal development (Livi, 1897, cited in du V. Florey, Reference du V. Florey1970; Miller & Hassanein, Reference Miller and Hassanein1971). A higher PI at birth suggests the presence of a well-functioning placenta and advanced fetal growth (Räsänen et al., Reference Räsänen, Kaprio, Laitinen, Winter, Koskenvuo and Laitinen2000). A relatively low PI indicates that soft tissue mass is lower than normal for the infant’s stage of skeletal development. A PI below the 10th percentile (Miller & Merritt, Reference Miller and Merritt1979) or the 10th percentile for gestational age at birth (Goldenberg et al., Reference Goldenberg, Cutter, Hoffman, Foster, Nelson and Hauth1990) is considered a proxy for IUGR and a correlate of perinatal morbidity (Martínez-Jiménez et al., Reference Martínez-Jiménez, Gómez-García, Gil-Campos and Pérez-Navero2020). An infant with a PI below the 3rd percentile for the population at birth (PI < 20.0) is deemed malnourished (Miller & Hassanein, Reference Miller and Hassanein1971).
The PI was used here to approximate early postnatal growth, represented by true change in the PI between birth and age 1 year and between birth and age 3 years, as derived from latent difference score (LDS) modeling. These periods capture important short- and long-term changes in early life growth. The first year of life is characterized by rapid changes in growth, especially for the most vulnerable preterm infants (Belfort et al., Reference Belfort, Rifas-Shiman, Sullivan, Collins, McPhee, Ryan, Kleinman, Gillman, Gibson and Makrides2011). The longer 3-year period encompasses the first 1000 days when programming effects are likely to occur. A significant mean decline in the PI during either of these periods would suggest early postnatal growth faltering. A significant increase in the PI during either period would suggest compensatory postnatal growth. Growth in HC was examined in the same periods, using parallel LDS models.
The present study had three specific objectives: 1) to assess the direction and significance of change in the PI and HC between birth and age 1 year and between birth and age 3 years in individuals born at ELBW, 2) to determine whether interindividual differences in intraindividual PI or HC change were significant over these time periods, and 3) to test for correlational associations between early PI or HC change and internalizing or externalizing behaviors at ages 8 years and 14 years.
Since mean weight-for-age z-scores in this cohort declined significantly in the first year relative to the 50th percentile for infants of this gestational age and were not fully restored to average levels by the third year (Saigal et al., Reference Saigal, Stoskopf, Streiner, Paneth, Pinelli and Boyle2006), the group mean for the PI was expected to decline over the first three years of life. HC is known to increase over the same time frame. Significant individual differences were anticipated in both measures, reflecting interindividual variability in intraindividual change during the periods of interest. Given the potential for growth-limiting factors to support the emergence of affective disorders, greater individual declines in the PI in the first three years of life were expected to predict higher levels of internalizing, but not necessarily externalizing, behaviors in childhood and adolescence. Conversely, greater increases in HC in the first three years were expected to predict lower levels of internalizing and externalizing behaviors.
Method
Participants
We examined a regional cohort of 179 individuals born at ELBW between 1977 and 1982 to parents living in Canada, who survived to hospital discharge. This group represented almost half (45%) of the original cohort of 397 infants born during this period. The survivors were born near the limit of viability for that time. Universal, government-insured health care was available to all at birth. Most were born to White mothers (89%). Within the cohort, 137 (60 male) ELBW survivors had birth measures of length as well as weight, allowing calculation of the PI at birth. The gestational age of these infants at birth ranged from 23 to 36 weeks (median = 27 weeks), with birth weights from 560 to 1000 g (M = 838 g). Within this group, 31 (23%) were classified as SGA, with birth weights <10th percentile for gestational age (Kramer et al., Reference Kramer, Platt, Wen, Joseph, Allen, Abrahamowicz, Blondel and Bréart2001). Thirty-eight (28%) had significant neurosensory impairments (NSI), diagnosed at age 3 years by a physician as cerebral palsy, blindness, deafness, hydrocephalus, microcephaly, or developmental delay (Saigal et al., Reference Saigal, Rosenbaum, Hattersley and Milner1989). Most (85%) received respiratory support, with nearly half (45%) receiving assisted ventilation for 30 days or more. The mean length of their initial hospitalizations was 78 days (range: 7 to 367 days). Participants in the present study (n = 137) did not differ from ELBW nonparticipants (n = 42) in birth weight, gestational age, distributions of sex, NSI, or SGA, antenatal steroid exposure, incidence of cerebral hemorrhage, presence of bronchopulmonary dysplasia (BPD) or duration of respiratory support, socioeconomic status (SES) at birth, or age of assessment in childhood or adolescence (ps > 0.12).
These studies were approved by the Ethics Committee of Chedoke-McMaster Hospitals, the Ethics Committee of Hamilton Health Sciences, and/or the Hamilton Health Science Ethics Review Board. All respondents or their parents provided written informed consent and received remuneration for their participation.
Measures
Predictors
The PI has been variously defined over the course of the past century, but its most common expression in infants is birth weight × 100/crown-heel length3, or, in children and adults, weight/height3. The PI provides critical information about the nutritional health of the newborn and is commonly used to index growth between birth and 2 years. The BMI (weight/height2) was not the focus of this study because the weight to height ratio of the BMI is not proportional during periods of dynamic growth (Burton, Reference Burton2007; Cole et al., Reference Cole, Bellizzi, Flegal and Dietz2000; Howe et al., Reference Howe, Tilling, Benfield, Logue, Sattar, Ness, Smith, Lawlor and Hernandez2010), when weight gains are exponential while increases in height are linear. In contrast, the PI is relatively stable throughout childhood and adolescence (Zaniqueli et al., Reference Zaniqueli, Oliosa, Neves, Pani, Martins, de Souza Peçanha, Barbosa, de Faria, de Oliveira Alvim and Mill2019), is independent of sex and ethnicity (Dombrowski et al., Reference Dombrowski, Berry, Johnson, Saleh and Sokol1994), requires no correction for age, and estimates body fat stores more accurately than does the BMI (between ages 8 and 29 years; Peterson et al., Reference Peterson, Su, Thomas, Heo, Golnabi, Pietrobelli and Heymsfield2017; Zaniqueli et al., Reference Zaniqueli, Oliosa, Neves, Pani, Martins, de Souza Peçanha, Barbosa, de Faria, de Oliveira Alvim and Mill2019). In this study, the PI was calculated at birth and ages 1, 2, and 3 years, corrected for the number of months premature (Saigal et al., Reference Saigal, Stoskopf, Streiner, Paneth, Pinelli and Boyle2006), to track overall growth during early development. The PI was also calculated at ages 8 and 14 years without correction for prematurity, since age corrections of less than 3 months are less critical for explaining developmental changes at these older ages (Figure 1a). Predictions of internalizing and externalizing outcomes were tested in two series of LDS models of PI change to capture short- and long-term changes in the first 1000 days after conception (Barker, Reference Barker2012). As the first year of life was previously characterized by a precipitous drop in weight-for-age z-scores relative to the 50th percentile for infants of this gestational age (Saigal et al., Reference Saigal, Stoskopf, Streiner, Paneth, Pinelli and Boyle2006), we wished to model this change using a proportional measure of body size. We also wished to model change in the PI from birth to age 3 years, to investigate potential effects on later mental health outcomes of PI change within the longer time frame for programming effects.
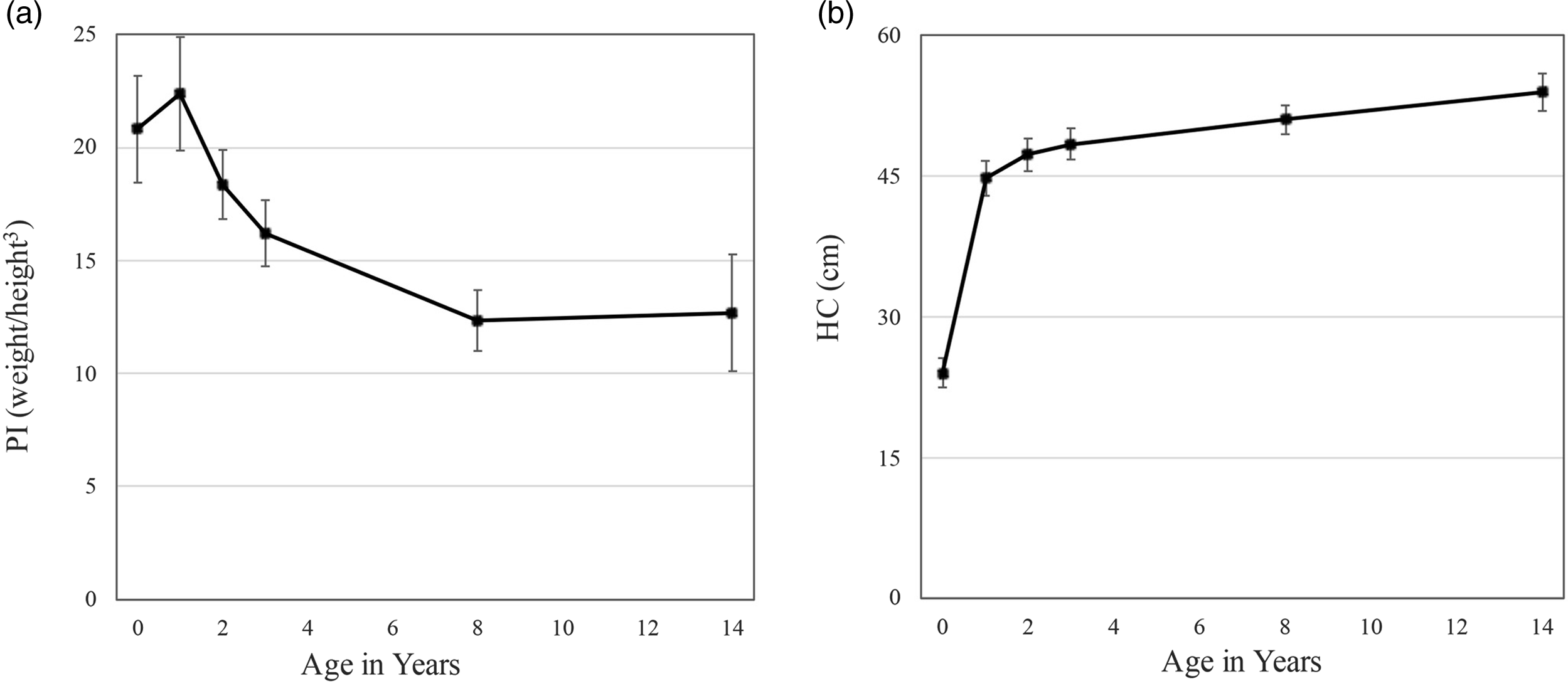
Figure 1. Change in mean levels of a) the ponderal index (PI) and b) head circumference (HC) between birth and adolescence in ELBW survivors.
Unlike change in the PI, change in HC is unidirectional, providing an alternate, linear measure of growth. Predictions of internalizing and externalizing were tested in LDS models of HC growth for the same periods examined for PI change.
Mental health outcomes
Full details of the original studies of childhood and adolescent psychopathology in this cohort have been described previously (Saigal et al., Reference Saigal, Pinelli, Hoult, Kim and Boyle2003; Szatmari et al., Reference Szatmari, Saigal, Rosenbaum and Campbell1993) and are briefly summarized here. Parents of school-age children born at ELBW were more likely than parents of control children to report problems with ADHD, with few other differences. In adolescence, parents reported significantly higher scores for ADHD, overanxious behaviors, and depression in adolescents born at ELBW than in control adolescents born at NBW.
Middle childhood assessment. When the children were 8 years old (M = 7.8 years, SD = 0.4), parents (predominantly mothers) completed the Ontario Child Health Study Scales (OCHS, Offord et al., Reference Offord, Boyle, Szatmari, Rae-Grant, Links, Cadman, Byles, Crawford, Blum, Byrne, Thomas and Woodward1987; Szatmari et al., Reference Szatmari, Saigal, Rosenbaum and Campbell1993). Parents were asked to consider their child’s behavior during the previous six months. Items were scored as 0, not true, 1, somewhat or sometimes true, or 2, very true or often true. Higher scores reflected more problem behaviors. As the Child Behavior Checklist (CBCL, Achenbach & Edelbrock, Reference Achenbach and Edelbrock1983) provided the basic pool of items for the OCHS, both instruments assess similar behaviors. Individual item scores at 8 years of age were summed to generate the internalizing (α = 0.86) and externalizing behaviors (α = 0.91) scales.
Adolescent assessment. Parents completed the Ontario Child Health Study-Revised Scales (OCHS-R, Boyle et al., Reference Boyle, Offord, Racine, Fleming, Szatmari and Sanford1993) when their child was 14 years old (M = 13.9 years, SD = 1.6), assessing the child’s behavior during the previous 6 months (Saigal et al., Reference Saigal, Pinelli, Hoult, Kim and Boyle2003). Items were scored 0, never or not true, 1, somewhat or sometimes true, or 2, very or often true. Higher scores indicated more problem behaviors. Subscale scores were based on the criteria of the Diagnostic and Statistical Manual of Mental Disorders, Third Edition, Revised (DSM-III-R; Kobylski, Reference Kobylski1988). Three subscale scores (overanxious, α = 0.73, separation anxiety, α = 0.73, depression, α = 0.87), were summed to generate a higher-order internalizing scale score (α = 0.91). Three subscale scores (conduct problems, α = 0.76, ADHD symptoms, α = 0.90, oppositional defiance, α = 0.88) were summed to generate a higher-order externalizing scale score (α = 0.94).
Both instruments have demonstrated good psychometric properties in previous research and are able to distinguish between children and youth using and not using outpatient mental health services (Achenbach & Rescorla, Reference Achenbach and Rescorla2001; Boyle et al., Reference Boyle, Offord, Racine, Fleming, Szatmari and Sanford1993).
Covariates
Sex was added to the LDS models of PI and HC change to account for possible sex differences in fetal development (Hintz et al., Reference Hintz, Kendrick, Vohr, Kenneth Poole and Higgins2006; Månsson et al., Reference Månsson, Fellman and Stjernqvist2015) and later mental health outcomes (e.g., Costello et al., Reference Costello, Worthman, Erkanli and Angold2007; Hack et al., Reference Hack, Youngstrom, Cartar, Schluchter, Taylor, Flannery, Klein and Borawski2004). Given that low SES is a well-known source of chronic stress, maternal SES at the time of birth was also included in the models to account for potential effects of ongoing environmental adversity in postnatal life (Schlotz & Phillips, Reference Schlotz and Phillips2009). The two-factor Index of Social Position (Hollingshead, Reference Hollingshead1969), incorporating education level and occupational status of the family head at the time of the child’s birth, was used to measure SES on a scale ranging from 1 (highest SES level) to 5 (lowest SES level).
Analytic approach: latent difference score (LDS) models
A LDS model was used to assess change in the PI over time (Klopack & Wickrama, Reference Klopack and Wickrama2020; McArdle, Reference McArdle2009; Newsom, Reference Newsom2015). In the first analysis, the observed PI at age 1 year was regressed on the PI at birth, and the difference between the two was modeled by a latent change factor. This modeling was achieved by fixing the loading to 1.0 for two pathways: a) the autoregressive pathway between the PI at birth and age 1 year, and b) the pathway between the latent PI change factor and the PI at age 1 year, thus capturing the “difference” variance in the latent change PI factor. For identification reasons, the intercept and residual of the PI at 1 year was fixed at zero in each model. In a second analysis, this same model specification was used to capture a LDS for change between birth and age 3 years. Ultimately, the latent change PI factor was characterized by a mean that represented the average of individual increases or decreases in the PI over time, and a variance that represented the individual differences in mean-level changes in the PI. The effect of birth PI on the PI change factor was included in each model to account for a possible proportional change effect (regression to mean), a statistical phenomenon that can give rise to misinterpretation of change estimates. In a third and fourth analysis, we assessed parallel LDS models for change in HC between birth and age 1 year, and between birth and age 3 years, respectively. These models were specified in exactly the same manner as those for the PI.
We first examined whether the baseline LDS model showed significant interindividual differences in intraindividual change in the PI or HC (see Figure 2a) and whether interindividual differences in intraindividual change in the PI or HC might be predictive of later internalizing or externalizing behaviors measured at middle childhood and adolescence (see Figure 2b and 2c). Conventional fit statistics were used to assess good or adequate model fit, including χ2 associated with a p > 0.05, a RMSEA of ≤ 0.060 or ≤ 0.080, a CFI of ≥ 0.950 or ≥ 0.900, and a SRMR of ≤ 0.050 or ≤ 0.080, respectively (Hooper et al., Reference Hooper, Coughlan and Mullen2008).
Figure 2. Latent difference score (LDS) modeling of change in the ponderal index (PI) during the first 3 years of life for a) the conditional baseline, b) internalizing behaviors in adolescence, and c) the internalizing subscales in adolescence (N = 137).
Data loss and missing data
Missing PI and mental health outcome data were noted for each assessment period after birth, due to intermittent participation across the six waves of this study (PI missingness at birth = 0%, age 1 year = 15%, age 2 years = 27%, age 3 years = 34%, middle childhood = 15%, adolescence = 18%; HC missingness at birth < 1.0%, age 1 year = 15%, age 2 years = 28%, age 3 years = 40%, middle childhood = 16%, adolescence = 20%; internalizing missingness in middle childhood = 14%, adolescence = 19%; externalizing missingness in middle childhood = 13%, adolescence = 20%). Our assumption was that missing data might be related to the health status of our participants, linking the PI index of neonates to vulnerabilities that could have impeded their consistent participation over time. Consequently, we tested for nonignorable missing data, more commonly known as not missing at random (NMAR) data, which occurs when an unobserved outcome variable (e.g., missing values not recorded for the outcome) is correlated with its corresponding outcome. We ran simple regression models, controlling for sex and SES at birth, to discover that missingness in the PI was significantly related to PI values at that same age (p < 0.05). Likewise, missingness in internalizing behaviors in middle childhood and adolescence was significantly associated with internalizing behaviors at the corresponding age, respectively (p < 0.05), and missingness in externalizing behaviors in middle childhood and adolescence was significantly associated with externalizing behaviors at the corresponding age, respectively (p < 0.05).
We accounted for NMAR in PI and internalizing outcomes using a “full-data” likelihood analysis that included missing data indicators (Enders, Reference Enders2013; Muthén et al., Reference Muthén, Asparouhov, Hunter and Leuchter2011). We chose the Diggle-Kenward (1994) selection model to incorporate binary missing data indicators (0 = observed, 1 = missing) into the LDS model. Using this framework, for example, both the PI at birth and at age 1 year predicted the age 1 missing data outcome in a logistic regression designed to account for nonignorable missingness. A missing indicator for internalizing or externalizing behaviors was added to the overall model that corresponded to the age of the mental health outcome being investigated. Missingness in the PI at birth to age 3 years LDS model was specified similarly (Figures S1a, S1b, and S1c). Furthermore, because missing data are believed to exist on a continuum between missing at random (MAR) and NMAR (Graham, Reference Graham2009) and normality assumptions are difficult to ascertain in both situations, we reran our analyses without missing data indicators under a MAR assumption. All analyses were conducted in Mplus 8.7 (Muthén & Muthén, Reference Muthén and Muthén2017), with robust full information maximum likelihood (MLR) estimation that does not need to assume multivariate normality (Yuan & Bentler, Reference Yuan and Bentler1998). MLR estimates can also accommodate non-independence among the observations.
Results
Descriptive statistics
As the ELBW sample size (N = 137) was relatively small for structural equation modeling (SEM), we limited our investigations to examining a simple LDS model with observed variables that estimated few parameters. Mean levels, standard errors, skew, kurtosis, and range of observed values for all study variables are presented in Table 1. Univariate normality was observed for all variables (within cut-off values ± 2 for skew and ± 7 for kurtosis, Byrne, Reference Byrne2010). In this sample, 20% (n = 23) of participants had elevated childhood internalizing scores, indicating significant concern or clinical disorder (sex-specific T-scores ≥ 64), and 18% (n = 21) had elevated externalizing scores (Achenbach, Reference Achenbach1991). In adolescence, 28% (n = 31) had internalizing scores indicating significant concern and 28% (n = 31) had elevated externalizing scores. The same sex-specific evaluation was applied in adolescence with a slightly more stringent criterion (T-scores ≥ 65), in line with recent practical guidelines for T-score interpretation (e.g., de Beurs et al., Reference de Beurs, Boehnke and Fried2022). At each assessment, variances in internalizing and externalizing scores were similar across groups.
Table 1. Descriptive statistics for study variables (N = 137; 43.8% male)
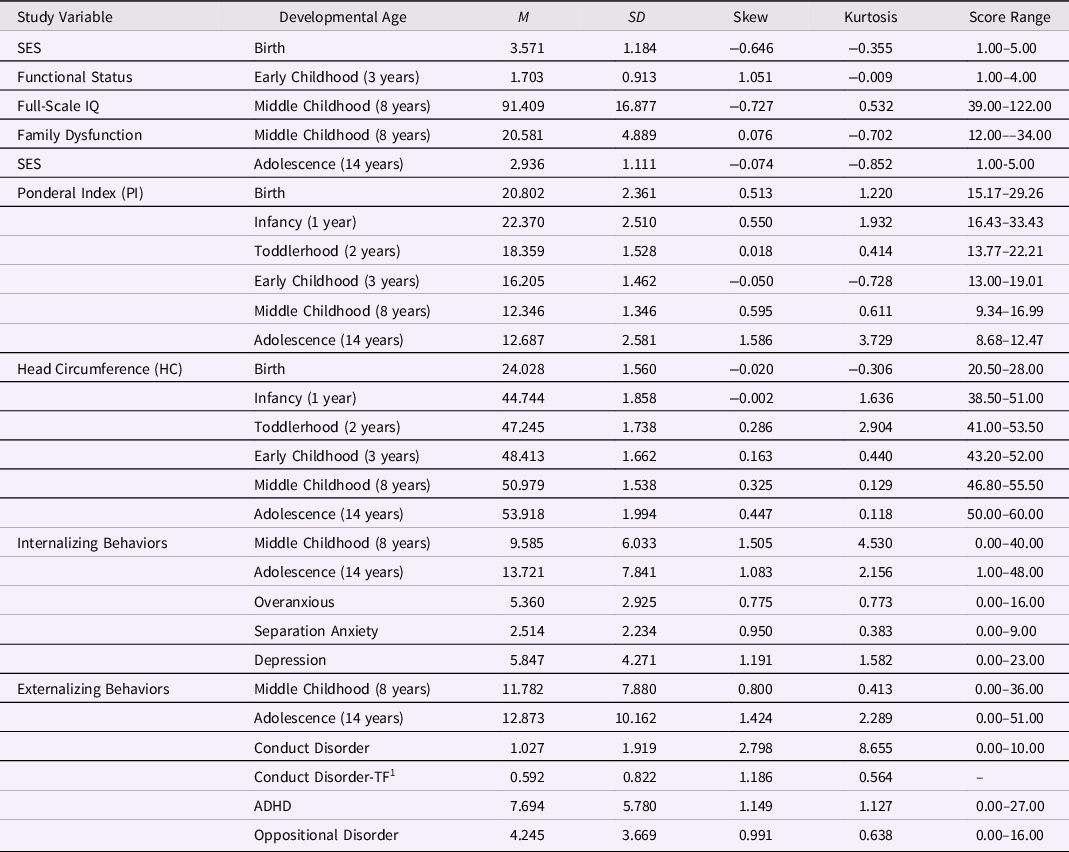
Note. SES represents socioeconomic status (highest SES level = 1; lowest SES level = 5); functional status refers to level of dysfunction (none, mild, moderate, severe); the ponderal index (PI) was measured as weight(kg)/height(m3).
1 Conduct disorder was skewed, thus, data for Conduct Disorder were square-root transformed (TF) in the models with externalizing subtypes.
Preliminary analyses
Due to concerns about the influence of nonignorable missingness on our findings, the PI conditional baseline and predictive models were all analyzed under conditions of missingness as MAR and NMAR (see supplementary materials for NMAR models). The findings were similar in statistical significance, strength, and direction of effects for both MAR and NMAR models, for internalizing and externalizing behaviors. For this reason, we chose to report only the values from the MAR analysis.
Latent change in the ponderal index (PI) in predicting later mental health outcomes
Change in PI: birth to 1 year
In a first step, we ran a conditional baseline LDS model to assess change in the PI in the first year, controlling for sex, and found good global fit between the sample data and implied model (Table 2). The residual for the PI change factor was statistically significant, p < 0.001, indicating that there were substantial individual differences in intraindividual change in PI during the first year. (The intercept of the PI latent change variable was not interpreted in any of the following analyses because a value of zero change is meaningless). Importantly, the PI at birth was significantly associated with change in the PI, indicating that lower levels of the PI at birth were associated with larger increases in the PI during the first year, β = −0.648, p < 0.001 (Table 3; ΔPI = 1.568). Thus, thinner neonates showed greater gains in the PI during the first year. The PI change factor was not influenced by sex.
Table 2. Model fit for the ponderal index (PI) latent difference score (LDS) models (birth to age 1 year): baseline and predicting later internalizing behaviors
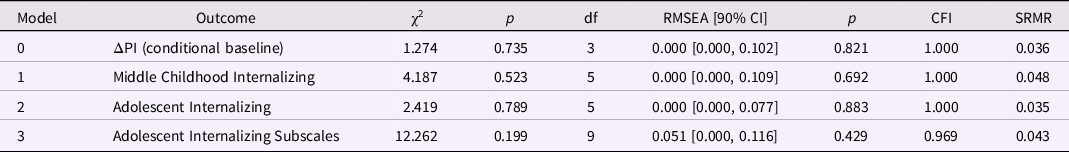
Note. ΔPI is the latent change in the ponderal index between birth and age 1 year; the baseline model is ΔPI conditional on sex; the three remaining models included ΔPI and SES at birth as predictors of later internalizing behaviors.
Table 3. Standardized estimates for the conditional baseline ponderal index (PI) latent difference score (LDS) model and PI change from birth to age 1 year in LDS models predicting internalizing behaviors
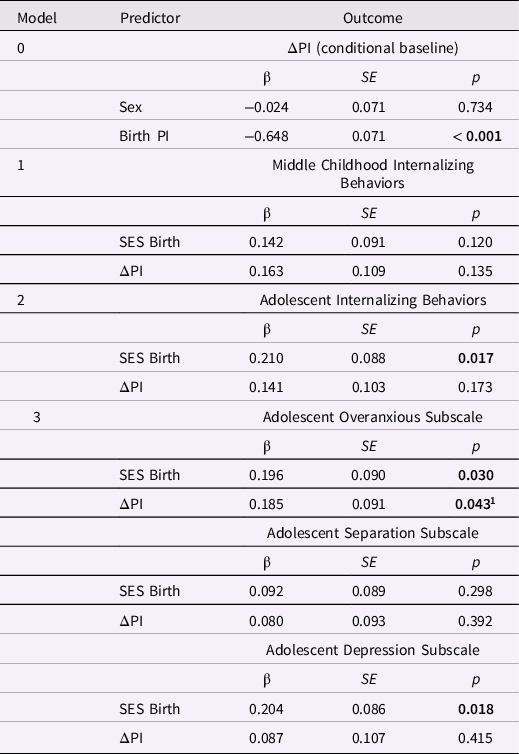
Note. Δ PI is the latent change in the ponderal index between birth and age 1 year. The baseline model is Δ PI conditional on sex; the three remaining models included Δ PI and SES at birth as predictors of later internalizing behaviors. Greater ΔPI values indicate more positive change in PI. The highest SES level = 1; the lowest SES level = 5.
1 The association between ΔPI and the adolescent overanxious subscale (p = .043) attained significance when using standardized data; in unstandardized results, p = .055.
Next, we tested whether change in the PI between birth and age 1 year predicted mental health outcomes (middle childhood and adolescent internalizing or externalizing behaviors, and the adolescent subscales), controlling for SES and sex.
Internalizing behaviors and PI change from birth to age 1 year. All three predictive models incorporated the baseline model and had good global fit (Table 2). Increases in PI between birth and age 1 year did not predict internalizing behaviors in middle childhood or adolescence (Table 3). However, testing the overanxious, separation anxiety, and depression subscales together in one analysis revealed that positive change in the PI predicted higher overanxious problems, β = 0.185, p = 0.043 (but not separation anxiety or depression; Table 3). In other words, after accounting for the baseline association, relatively larger increases in PI over the first year of postnatal life were associated with higher levels of generalized anxiety in adolescence. Lower SES at birth was associated with higher internalizing, overanxious, and depression scores in adolescence, ps < 0.04.
Externalizing behaviors and PI change from birth to age 1 year. Only the model predicting the externalizing subscales had good global fit (Table S1). Testing the conduct, ADHD, and oppositional behaviors together revealed no associations with PI change. Lower SES at birth was associated with higher levels of conduct problems and ADHD in adolescence, ps < 0.01 (Table S2).
Change in PI: birth to 3 years
To assess PI change between birth and the first 3 years of postnatal life, we ran a conditional baseline LDS model, controlling for sex, and found good fit between the sample data and implied model (Table 4). Individual differences in intraindividual change in PI during this period were statistically significant, p < 0.001. Again, PI at birth predicted the PI change factor, but this time lower PI at birth predicted smaller increments of negative change, namely, smaller decreases in the PI during the first three years of postnatal life, β = −0.838, p < 0.001 (Table 5; ΔPI = −4.597). Thus, thinner neonates showed smaller declines in the PI over 3 years. Sex was unrelated to the PI change factor.
Table 4. Model fit for the ponderal index (PI) latent score difference (LDS) models (birth to age 3 years): baseline and predicting later internalizing behaviors
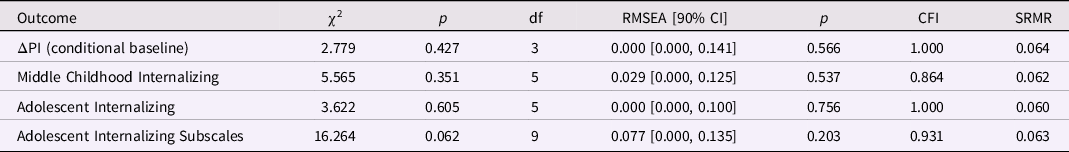
Note. ΔPI is the latent change in the ponderal index between birth and age 3 years; the baseline model is ΔPI conditional on sex; the three remaining models included ΔPI and SES at birth as predictors of later internalizing behaviors.
Table 5. Standardized estimates for the conditional baseline ponderal index (PI) latent difference score (LDS) model and PI change from birth to age 3 years in LDS models predicting later internalizing behaviors
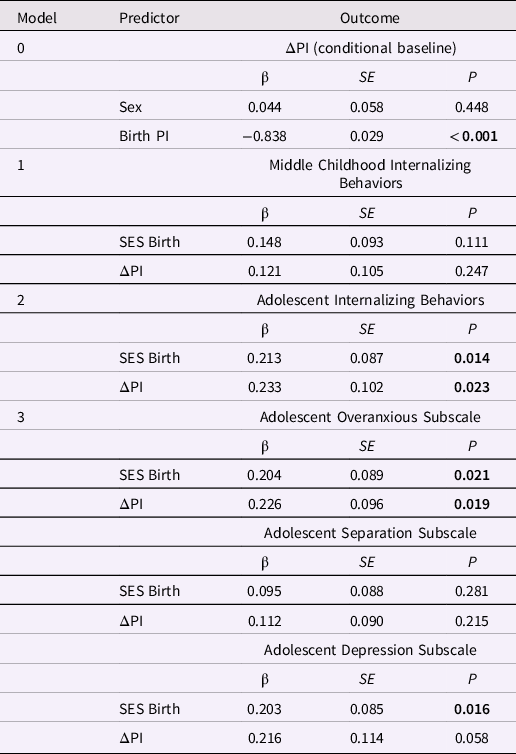
Note. ΔPI is the latent change in the ponderal index between birth and age 3 years. The baseline model is ΔPI conditional on sex; the three remaining models included ΔPI and SES at birth as predictors of later internalizing behaviors. Greater ΔPI values indicate less negative change in PI. The highest SES level = 1; the lowest SES level = 5.
Next, we tested whether change in the PI between birth and the first 3 years of postnatal life predicted later mental health outcomes (middle childhood internalizing or externalizing behaviors, adolescent internalizing or externalizing behaviors, and the adolescent subscales).
Internalizing behaviors and PI change from birth to age 3 years. All three predictive models included the baseline model. Global fit was good in the models predicting adolescent internalizing behaviors (Table 4). Change in PI between birth and age 3 years predicted internalizing behaviors in adolescence, β = 0.233, p = 0.023 (Table 5). Specifically, smaller decreases in PI over the first 3 years of postnatal life were associated with higher levels of internalizing behaviors in adolescence. Smaller decreases in the PI were also associated with higher overanxious scores, β = 0.226, p < 0.019 (but not separation anxiety or depression scores, ps > 0.055; Table 5). Thus, thinner neonates (who showed smaller decreases in the PI over three years) displayed higher levels of internalizing behaviors in adolescence, especially overanxious problems. Lower SES at birth was associated with higher internalizing, overanxious and depression scores in adolescence, ps < 0.03. Additional models (e.g., PI change between birth and two years or between 1 and 3 years) provided no new or conflicting information.
Externalizing behaviors and PI change from birth to age 3 years. Only the model predicting the externalizing subscales had adequate global fit, apart from a high SRMR statistic (0.101; Table S3). Findings from this model were similar to those of the LDS model of PI change between birth and age 1 year, showing no associations between PI change and any of the externalizing subscales. Lower SES at birth was associated with higher levels of conduct problems and ADHD in adolescence, ps < 0.02 (Table S4).
Latent change in head circumference (HC) in predicting later mental health outcomes
Change in HC: birth to 1 year
Most fit indices indicated adequate global fit for the baseline LDS model assessing HC change between birth and age 1 year, except for a low CFI, 0.844, suggesting that the average correlation among the variables was small (Table 6). Individual differences in intraindividual change in HC during this period were statistically significant. Like the PI, HC at birth was significantly associated with change in HC, β = −0.595, p < 0.001, indicating that smaller HC at birth was associated with greater increases in HC during the first year (Table 7; ΔHC = 20.72). Male sex was also associated with greater increases in HC, β = −0.246, p < 0.001. (Table 7).
Table 6. Model fit for the head circumference (HC) latent difference score (LDS) models (birth to age 1 year): baseline and predicting later internalizing behaviors
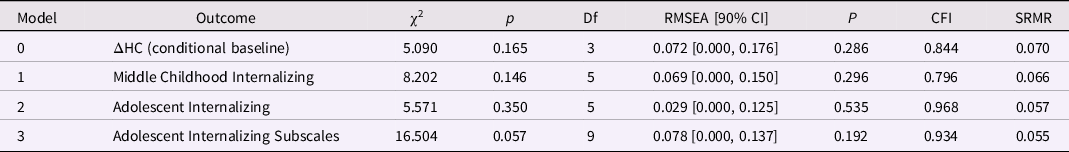
Note. ΔHC is the latent change in head circumference between birth and age 1 year; the baseline model is ΔHC conditional on sex; the three remaining models included ΔPI and SES at birth as predictors of later internalizing behaviors.
Table 7. Standardized estimates for the conditional baseline head circumference (HC) latent difference score (LDS) model and HC change from birth to age one year in LDS models predicting later internalizing behaviors
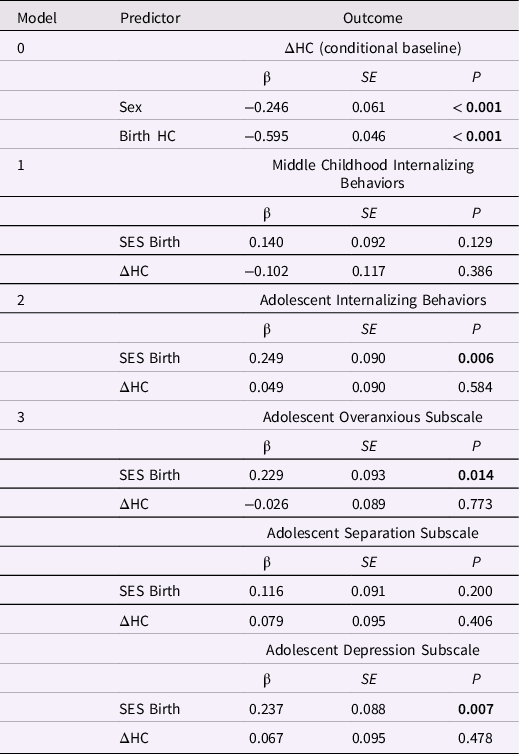
Note. ΔHC is the latent change in head circumference between birth and age one year. The baseline model is ΔHC conditional on sex; the three remaining models included ΔHC and SES at birth as predictors of later internalizing behaviors. Greater ΔHC values indicate more positive change in HC. The highest SES level = 1; the lowest SES level = 5.
Internalizing behaviors and HC change from birth to age 1 year. Only the models of adolescent internalizing behaviors and internalizing subscale scores had adequate global fit. (Table 6). However, change in HC between birth and age 1 year was not associated with adolescent internalizing or the subscale scores, ps > 0.40 (Table 7). Lower SES at birth was associated with higher levels of adolescent internalizing behaviors, overanxious behaviors, and depression, ps < 0.02.
Externalizing behaviors and HC change from birth to age 1 year. Only the model predicting the externalizing subscales had good global fit (Table 8). Greater increases in HC between birth and age 1 year predicted higher ADHD scores in adolescence, β = 0.270, p = 0.010 (but not conduct or oppositional behavior; Table 9). Lower SES at birth was associated with higher levels of conduct problems and ADHD in adolescence, ps < 0.01.
Table 8. Model fit for the head circumference (HC) latent difference score (LDS) models (birth to age one year): baseline and predicting later externalizing behaviors
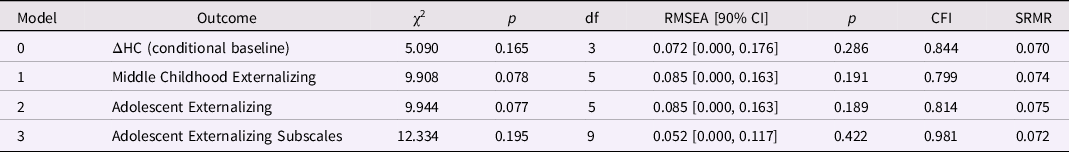
Note. ΔHC is the latent change in head circumference between birth and age one year. The baseline model is ΔHC is conditional on sex; the three remaining models included ΔHC and SES at birth as predictors of later externalizing behaviors.
Table 9. Standardized estimates for the conditional baseline head circumference (HC) latent difference score (LDS) model and HC change from birth to age one year in LDS models predicting later externalizing behaviors
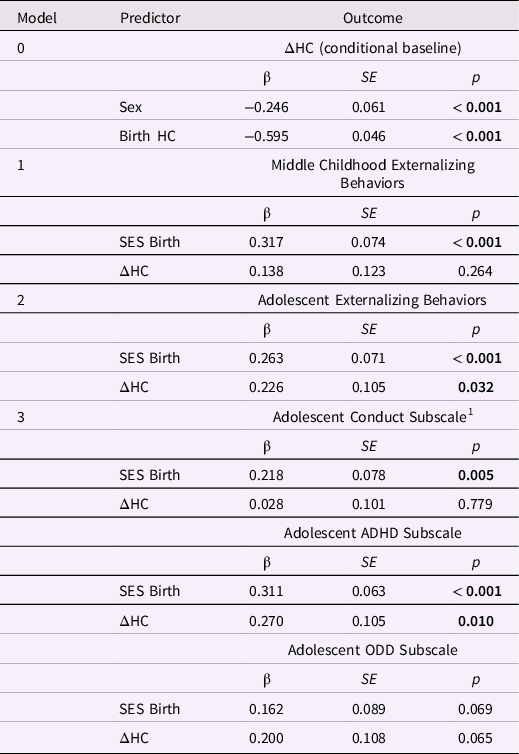
Note. ΔHC is the latent change in head circumference between birth and age one year; ADHD is attention deficit and hyperactivity disorder; ODD is oppositional defiant disorder. The baseline model is ΔHC conditional on sex; the three remaining models included ΔHC and SES at birth as predictors of later externalizing behaviors. Greater ΔHC values indicate more positive change in HC. The highest SES level = 1; the lowest SES level = 5.
1 Conduct disorder was skewed, thus, the model with externalizing subtypes as outcomes included data for Conduct Disorder square root transformed.
Change in HC: birth to 3 years
The baseline LDS model capturing HC change between birth and age 3 years, controlling for sex and SES, had good global fit (Table S5). Like the PI, HC at birth was significantly associated with change in HC to age 3 years, β = −0.659, p < 0.001, indicating that smaller HC at birth was associated with greater increases in HC during the first 3 years (Table S6; ΔHC = 23.22 cm). Male sex was also associated with greater increases in HC, β = −0.258, p < 0.001 (Table S6).
Internalizing behaviors and HC change from birth to age 3 years. All the predictive models for internalizing measures had good global fit (Table S5). Findings from these models were similar to those of the LDS models of HC change between birth and age 1 year, showing no associations between HC change and internalizing behaviors. Lower SES was associated with higher levels of internalizing behaviors, overanxious behaviors and depression in adolescence, ps < 0.02 (Table S6).
Externalizing behaviors and HC change from birth to age 3 years. Only the model predicting the externalizing subscales had acceptable global fit (Table S7). Smaller HC at birth was associated with larger increases in HC during the first 3 years, β = −0.659, p < 0.001. Change in HC did not predict any of the externalizing subscales in adolescence (Table S8). Lower SES was associated with higher levels of conduct problems and ADHD in adolescence, ps < 0.02.
Summary of findings
All of the baseline models showed significant regression to the mean. Thinner neonates showed greater increases in the PI during the first year, and smaller decreases in the PI over 3 years.Footnote 1 Similarly, neonates with relatively smaller heads showed greater HC growth during the first year and over 3 years. It is only after controlling for the starting (birth) values that change in the PI or HC predicted internalizing behaviors or ADHD, respectively. Relatively higher PI values at the end of each period predicted increased internalizing behaviors in adolescence, particularly overanxious behavior. Relatively greater HC at the end of the first year of life predicted greater ADHD problems in adolescence.
Comparisons with normative data
To provide context for the PI and HC changes in ELBW infants, we used the 50th percentile Center for Disease Control (CDC) reference norms for weight, height, and HC, averaged across sex, to calculate values of the PI and HC for average-sized, term-born infants (www.cdc.gov; 2000). The mean for the PI at birth in term-born infants born at normal weight was 28.333 kg/m3, well above the ELBW mean of 20.802 kg/m3. In contrast to ELBW infants, the PI in term-born infants exhibited a substantial decrease beginning in the first year (CDC norms; −4.72) as the rate of weight gain decelerated relative to height3. The decrease in PI continued over the next two years (CDC norms, total decline; −11.86). In sum, the trajectory of the PI showed marked directional differences between ELBW and term-born children in the first year, but by age 3 years, mean levels of the PI had converged across groups (CDC norms: M = 16.47, ELBW: M = 16.21; Figure 3a).
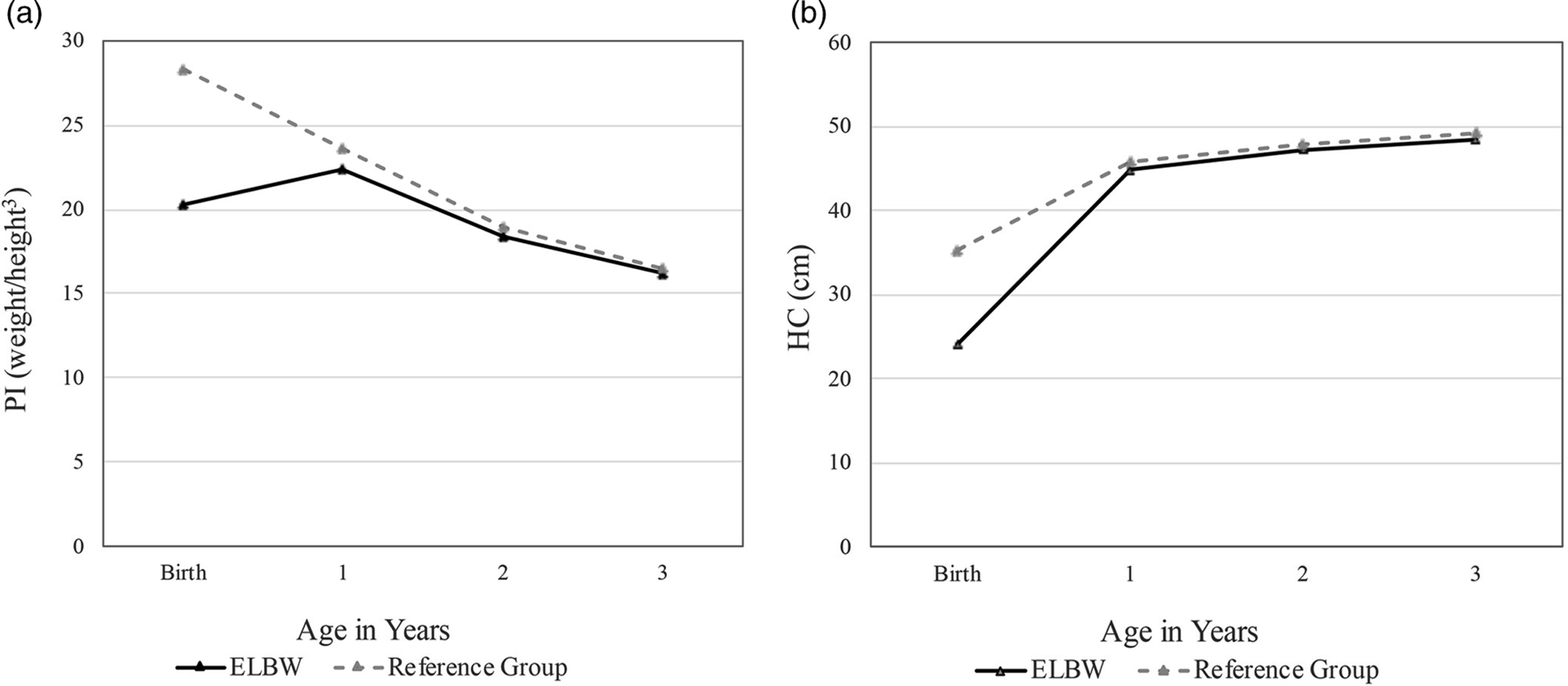
Figure 3. Change in a) the ponderal index (PI) and b) head circumference (HC) between birth and age 3 years in ELBW children and NBW children, derived from Center for Disease Control (CDC) norms at the 50th percentile.
Mean HC at birth in the term-born group (35.26 cm) was 11.23 cm (47%) larger than mean HC in the ELBW group (24.03 cm). The upward trajectory of HC growth in the first year was considerably steeper for ELBW than term-born children, but by age 3 years, mean HC had converged across groups (CDC norms: M = 49.16 cm, ELBW: M = 48.41 cm; Figure 3b).
Sensitivity analyses
Because factors such as disability, cognitive limitations, family dysfunction, or financial duress during development may influence the emergence and maintenance of adolescent mental health problems, we tested the significant associations between PI change (birth to 1 year, birth to 3 years) and the adolescent internalizing sub-scales, and between HC change (birth to 1 year) and the adolescent externalizing subscales for sensitivity to each of these factors in separate mediation models (Figure S2). None of the new models indicated significant mediation by any of these factors. Generally, the mediation models with functional status (disability), IQ, or concurrent SES at adolescence had poor fit. The remaining factor, childhood family dysfunction, did not mediate between PI change and the adolescent internalizing subscales, or between HC change and the adolescent externalizing subscales, in models that fit well. Further, adding family dysfunction to these models failed to alter the original relations between growth parameters and mental health outcomes. (For an example, see results from the model for PI change and the internalizing subscales with family dysfunction as the mediator in Tables S9, S10).
Discussion
Anthropomorphic indicators such as weight, length, and HC serve as surrogate indicators of nutrient accretion during the critical first weeks of life (Belfort & Ramel, Reference Belfort and Ramel2019). The primary aim of this study was to test whether early growth parameters in individuals born at ELBW were predictive of internalizing or externalizing behaviors at later stages of development. Importantly, the baseline models indicated that change in the PI or HC was significantly associated with the PI or HC values at birth. ELBW infants who showed greater overall weight gains during the first 3 years were relatively thinner at birth than other ELBW infants, and those showing greater head growth were likely to have had smaller HC at birth. Thus, infants who were more vulnerable at birth showed relatively greater catch-up growth in the PI and HC during this early period.
Second, the predictive models revealed specific associations between change in the PI and internalizing measures, and between growth in HC and ADHD behaviors. With the baseline relations accounted for, greater increases in the PI during the first year positively predicted anxiety in adolescence. Similarly, smaller net decreases in the PI during the first 3 years predicted higher levels of teen internalizing behaviors and anxiety. These associations suggested that adolescent internalizing levels were associated with relatively greater weight gain in the first year and rates of weight gain that remained high at age 3 years. Relatively greater growth in HC during the first year predicted higher levels of ADHD problems in adolescence. There were no reliable relations between the early growth parameters and childhood internalizing, largely because of poor model fit in these analyses.
With respect to the covariates, baseline models indicated that male sex was associated with larger HC (Galjaard et al., Reference Galjaard, Ameye, Lees, Pexsters, Bourne, Timmerman and Devlieger2019), whereas the PI did not differ by sex. Low maternal SES was associated with overanxious symptoms, depression, conduct problems, and ADHD (but not separation anxiety or oppositional defiant disorder), in line with recent studies (Markham & Spencer, Reference Markham and Spencer2022; McLaughlin et al., Reference McLaughlin, Breslau, Green, Lakoma, Sampson, Zaslavsky and Kessler2011). Additional models provided no evidence to suggest that significant relations in the predictive models were mediated by functional status, IQ, childhood family dysfunction, or concurrent SES.
Internalizing behaviors following adaptation to nutritional deficit
What explains the relations between change in the PI and internalizing behaviors in adolescence? These paradoxical findings invite closer scrutiny and discussion.
To begin with, the basal energy requirements of very preterm neonates are higher than those of babies born closer to term (Embleton et al., Reference Embleton, Pang and Cooke2001), and early intolerance for enteral feeding may exacerbate the risk of growth faltering before term-equivalent age. As a result, these infants tend to experience significant protein and energy deficits in the first weeks of life, with incidence rates ranging from 43 to 97% even in recent decades (Horbar et al., Reference Horbar, Ehrenkranz, Badger, Edwards, Morrow, Soll, Buzas, Bertino, Gagliardi and Bellù2015; Su, Reference Su2014). Recouping these losses requires nutrient intake that exceeds the requirements for normal maintenance and growth (Cooke, Reference Cooke2010; Embleton et al., Reference Embleton, Pang and Cooke2001; Heird, Reference Heird1999). Nonetheless, most preterm infants (80%) show some evidence of catch-up growth in weight, length, and HC, beginning in the first few months of postnatal life (Euser et al., Reference Euser, De Wit, Finken, Rijken and Wit2008; Hack et al., Reference Hack, Schluchter, Margevicius, Andreias, Taylor and Cuttler2014; Hellström et al., Reference Hellström, Sigurdsson, Löfqvist, Hellgren and Kistner2020). By the end of the second year, variable intraindividual growth rates appear to stabilize and growth begins to follow an infant’s genetically guided trajectory (Ong et al., Reference Ong, Ahmed, Emmett, Preece and Dunger2000).
Any intraindividual change in the PI denotes a shift in the relative contributions of body weight and body length to the index. For ELBW newborns with significant energy and protein deficits, preferentially directing available nutritional resources to weight gain may be an adaptive defensive response. Given their higher metabolic needs and poor nutritional intake (Heird, Reference Heird1999), it may be more adaptive for these newborns to invest energy in maintaining energy stores rather than diverting resources to physical development during the critical early months of life. The latter strategy could risk depleting energy levels below a threshold required for survival, especially for preterm infants who are sicker (Ehrenkranz et al., Reference Ehrenkranz, Younes, Lemons, Fanaroff, Donovan, Wright, Katsikiotis, Tyson, Oh, Shankaran, Bauer, Korones, Stoll, Stevenson and Papile1999; Ramel et al., Reference Ramel, Brown and Georgieff2014), more immature (Pridham et al., Reference Pridham, Brown, Sondel, Clark and Green2001), or more sensitive to the type of nutrition available (Barker et al., Reference Barker, Forsén, Uutela, Osmond and Eriksson2001).
A similar defensive strategy has been documented in humans and animals exposed to famine; the metabolic stress elicited by starvation initiates multiple hormonal, autonomic, and metabolic changes to promote the accumulation and storage of fat (Schwartz & Seeley, Reference Schwartz and Seeley1997). Related evidence is seen in the offspring of women who were exposed as fetuses to the Dutch Hunger Winter in 1944–45. Famine exposure in utero resulted in shorter newborns in the next generation, while average birth weight was preserved, thereby increasing the PI (Painter et al., Reference Painter, Osmond, Gluckman, Hanson, Phillips and Roseboom2008). Nutritional deficits that influence height growth are also malleable in the other direction: when nutritional deficits are ameliorated by enhanced early parenteral (intravenous) and enteral nutrition, stature may be increased (Loÿs et al., Reference Loÿs, Maucort-Boulch, Guy, Putet, Picaud and Haÿs2013). Notably, the influence of famine exposure extends beyond physical growth: famine exposure in early life is reported to have deleterious effects on adult mental health, for example, increasing the incidence of affective disorders following the Dutch famine (Brown et al., Reference Brown, van Os, Driessens, Hoek and Susser2000), and elevating the overall risk of mental health problems following the Great Leap Forward famine in China, 1959–61 (Huang et al., Reference Huang, Phillips, Zhang, Zhang, Shi, Song, Ding, Pang and Martorell2013).
Some infants in the present ELBW cohort were undernourished at birth, given that PI values ranged from 15 to 29. In accord with the practices of the era, most of the cohort received intravenous lipids and amino acids (M = 24 and 25 days, respectively) and concentrated preterm formula (SMA24, SMA30 calories/ounce) during their initial hospitalization. With enhanced nutrition, first-year growth in the cohort was exponential, leading to mean increases of 823% in weight and 105% in height, and an increase in the PI from 20.8 at birth to 22.4 by 12 months. After the first year, gains in weight and height became more balanced, leading to reductions in the PI: by the end of the second year, growth rates had slowed considerably for both weight and height (M PI = 18.36). PI values were further reduced by the end of the third year (M PI = 16.21), by which time they showed parity with those of typically developing infants (cf. similar trajectories in Hellström et al., Reference Hellström, Sigurdsson, Löfqvist, Hellgren and Kistner2020).
While the decline in mean levels of the PI from birth seen in full-term infants appears to be normative (Figure 3a), given the undernourished status of some ELBW neonates, it is uncertain how far their PI values could decline from birth without risking their survival. Conversely, modeling indicated that ELBW infants who were thinner at birth showed more pronounced increases in the PI during the first year and smaller declines in the PI over the first 3 years. These changes reflect adjustments in the allocation of resources to weight versus height during early postnatal growth. Although speculative, this pattern of adaptation seems consistent with biological priorities that favor postnatal weight gain over physical development. It is important to note that internalizing behaviors in ELBW adolescents were predicted by the dynamic changes in the weight/height ratio that occurred during an early critical period for postnatal development, rather than by lower absolute values of birth weight or thinness (lower PI) at birth (data not shown).
Altered brain development following nutritional deficit
According to theories of early programming, adaptations that support immediate survival may incur costs to later physical health (Gluckman et al., Reference Gluckman, Hanson, Cooper and Thornburg2008; Metcalfe & Monaghan, Reference Metcalfe and Monaghan2001). Such costs may extend to mental health, but what mechanism would explain this effect? Potential growth-limiting factors in the prenatal environment include IUGR and maternal health characteristics such as smoking during pregnancy, hypertensive disorders, and older maternal age (Sannoh et al., Reference Sannoh, Demissie, Balasubramanian and Rhoads2003). Postnatally, infant mental health may be affected by exposures to infection, painful procedures, and prolonged separation from the primary caregiver while in neonatal care (Drvaric et al., Reference Drvaric, Van Lieshout and Schmidt2013) or parental distress related to caring for a highly vulnerable infant (Pierrehumbert et al., Reference Pierrehumbert, Nicole, Muller-Nix, Forcada-Guex and Ansermet2003). There is abundant neuroanatomical evidence to suggest that the normal neurodevelopmental trajectory may be permanently altered following very preterm birth (e.g., Guellec et al., Reference Guellec, Lapillonne, Marret, Picaud, Mitanchez, Charkaluk, Fresson, Arnaud, Flamand, Cambonie, Kaminski, Roze, Ancel, Larroque, Ancel, Blondel, Bréart, Dehan, Garel and Voyer2016; Hadaya & Nosarti, Reference Hadaya and Nosarti2020; Hedderich et al., Reference Hedderich, Bäuml, Berndt, Menegaux, Scheef, Daamen, Zimmer, Bartmann, Boecker, Wolke, Gaser and Sorg2019; Ment et al., Reference Ment, Kesler, Vohr, Katz, Baumgartner, Schneider, Delancy, Silbereis, Duncan, Constable, Makuch and Reiss2009; Narberhaus et al., Reference Narberhaus, Lawrence, Allin, Walshe, McGuire, Rifkin, Murray and Nosarti2009). Altered neurodevelopment during the perinatal period is likely to affect brain systems that are integral to mental health (e.g., Gale & Martyn, Reference Gale and Martyn2004; Nomura et al., Reference Nomura, Brooks-Gunn, Davey, Ham and Fifer2007; Pettersson et al., Reference Pettersson, Larsson, D’Onofrio, Almqvist and Lichtenstein2019; Smith et al., Reference Smith, Schmidt-Kastner, McGeary, Kaczorowski and Knopik2016).
Serotonergic regulation has long been associated with negative mood states (Cowen & Browning, Reference Cowen and Browning2015). As a neurotransmitter, brain serotonin is involved in processes that support experience-induced plasticity (Kojic et al., Reference Kojic, Dyck, Gu, Douglas, Matsubara and Cynader2000), synaptic modeling, and the refinement of neural connections during development (Manjarrez et al., Reference Manjarrez, Cisneros, Herrera, Vazquez, Robles and Hernandez2005). During gestation, increased metabolic stress may lead to disturbances in the metabolism of brain serotonin in growth-restricted infants (Manjarrez et al., Reference Manjarrez, Cisneros, Herrera, Vazquez, Robles and Hernandez2005). Alterations in serotonergic activity during critical periods of cortical differentiation may lead to neurodevelopmental anomalies that affect learning. In addition to its role in synaptic modeling, serotonin has important roles in regulating HPA-axis activity (Gotlib et al., Reference Gotlib, Joormann, Minor and Hallmayer2008; Gunnar & Quevedo, Reference Gunnar and Quevedo2007), cortisol responses (Reimold et al., Reference Reimold, Batra, Knobel, Smolka, Zimmer, Mann, Solbach, Reischl, Schwärzler, Gründer, Machulla, Bares and Heinz2008; 2011), and cardiovascular and behavioral stress reactivity (Davies et al., Reference Davies, Hood, Argyropoulos, Morris, Bell, Witchel, Jackson, Nutt and Potokar2006). Increased stress reactivity has been implicated in the pathogenesis of mood disorders in adulthood (Kajantie et al., Reference Kajantie, Feldt, Räikkönen, Phillips, Osmond, Heinonen, Pesonen, Andersson, Barker and Eriksson2007; Wüst et al., Reference Wüst, Entringer, Federenko, Schlotz and Hellhammer2005). Thus, poor fetal growth may also lead to disturbances in serotonergic activity that negatively influence stress regulation.
Growth in head circumference (HC) and ADHD
Apart from genetic predisposition, prematurity and low birth weight are the main risk factors for the development of ADHD, with greater risks for infants born extremely preterm or ELBW (Franz et al., Reference Franz, Bolat, Bolat, Matijasevich, Santos, Silveira, Procianoy, Rohde and Moreira-Maia2018). HC is a rough proxy for brain volume (Bartholomeusz et al., Reference Bartholomeusz, Courchesne and Karns2002; Belfort & Ramel, Reference Belfort and Ramel2019). Mean HC in this sample of ELBW infants at birth was substantially smaller than CDC norms for infants born at term. Smaller HC has been associated with increased risk of ADHD at school age in children born preterm (e.g., Soldateli et al., Reference Soldateli, Silveira, Procianoy, Belfort, Caye, Leffa, Franz, Barros, Santos, Matijasevich, Barros, Tovo-Rodrigues, Menezes, Gonçalves, Wehrmeister and Rohde2022). Term-born children diagnosed with ADHD around the time of school entry are also more likely to have had smaller HC and lower PI at birth, outcomes that are consistent with physiological adaptation in utero (Heinonen et al., Reference Heinonen, Räikkönen, Pesonen, Andersson, Kajantie, Eriksson, Vartia, Wolke and Lano2011; Lahti et al., Reference Lahti, Räikkönen, Kajantie, Heinonen, Pesonen, Järvenpää and Strandberg2006). Our data extend the findings to adolescence for infants born at ELBW: ELBW neonates with smaller HC at birth showed relatively greater HC growth during the first year, and these infants had higher levels of ADHD problems in adolescence.
Strengths and limitations
The present investigation is one of only a handful of studies that examines the PI and later mental health outcomes in individuals born at ELBW. Strengths of the study include careful construction of LDS change models and the use of MLR estimation, which incorporates all available information into the analysis model and provides estimates that are robust to multivariate nonnormality. In addition to demonstrating good model fit and adjusting for important covariates (sex, familial SES), LDS change models accounted for intermittent participation across the waves of the study. We tested the LDS models under both MAR and NMAR assumptions, in the latter case using a “full-data” likelihood analysis. In addition, latent variables such as ΔPI or ΔHC reflect both the magnitude of the observed changes and the specific risks that underlie the changes (Woo, Reference Woo2017). Finally, given the clear temporal order between infant levels of the PI and assessments of internalizing in middle childhood and adolescence, our findings are not merely observational but suggest potentially causal associations between infant growth and later mental health.
Some limitations should also be considered when interpreting these findings. The size and statistical power of this high-risk sample were limited by the size of the original cohort and attrition over time. We attempted to address this problem by utilizing simple LDS models and accounting for intermittent participation through NMAR analyses that included missing data indicators to account for missing data. Although study participants did not differ from nonparticipants in SGA status, key covariates, or important neonatal exposures, our sample was too small to allow meaningful evaluation of group differences in birth size related to prematurity (early birth) versus IUGR. Aside from this question, the sample size was large enough to reveal interpretable associations between PI or HC change and internalizing or ADHD problems in individuals born at ELBW.
Second, the lack of a typically developing control group born at NBW is a limitation. Although a control group of NBW children was added to the ELBW cohort when both groups were eight years old, measures of birth length and height before age eight were not available for these children, preventing calculation of their PI values in early postnatal life. For comparisons to term-born children, we calculated mean values for the PI and HC between birth and age 3 years using CDC reference data at the 50th percentile for weight, height, and HC. Comparing the PI and HC trajectories of ELBW and term-born infants revealed striking contrasts in their patterns of early growth, though their respective trajectories appeared to converge by age 3 years.
Third, this study used a historical cohort born four decades ago, which did not benefit from improvements in neonatal care available to younger cohorts. Although these ELBW infants were nourished according to standards current at the time, approaches to postnatal nutritional intervention have continued to evolve (e.g., Embleton & van den Akker, Reference Embleton and van den Akker2019). Replication would be helpful for assessing whether the present findings generalize to ELBW survivors who have experienced more advanced neonatal care. Our analyses could easily be repeated in larger and more contemporary cohorts, as the variables included in the LDS models were simple measures of weight and height, sex, and SES at birth. Our findings may also be relevant for modern contexts, given that the world-wide incidence of preterm birth remains high, advanced levels of care are less likely to be available in low-resource settings (Blencowe et al., Reference Blencowe, Krasevec, de Onis, Black, An, Stevens, Borghi, Hayashi, Estevez, Cegolon, Shiekh, Ponce Hardy, Lawn and Cousens2019), and the limit of preterm viability has been pushed to as early as 23–24 weeks’ gestational age (Stoll et al., Reference Stoll, Hansen, Bell, Walsh, Carlo, Shankaran, Laptook, Sánchez, Van Meurs, Wyckoff, Das, Hale, Ball, Newman, Schibler, Poindexter, Kennedy, Cotten, Watterberg, D’Angio, DeMauro, Truog, Devaskar and Higgins2015).
Finally, internalizing behaviors were evaluated by parents, using slightly different screening tools across assessments. Formal diagnoses of internalizing behaviors derived from structured clinical interviews were not available. However, the use of parent reports from both assessments provided consistency across the two visits. Moreover, parent reports from the adolescent assessment explained more of the variance in mental health problems than did teen self-reports (Saigal et al., Reference Saigal, Pinelli, Hoult, Kim and Boyle2003). The Achenbach scales are well-validated and designed to be comparable across development (Achenbach et al., Reference Achenbach, Ivanova, Rescorla, Turner and Althoff2016), making them ideal for use in longitudinal studies. The problem subscales from the Achenbach and OCHS-R scales also show a high degree of overlap. We believe the discrepancy between findings from the childhood and adolescent LDS models was not likely to stem from the use of these two slightly differing scales but may have been related to lower levels of problem behaviors in childhood observed in the sample. Except for ADHD problems, childhood means for internalizing and externalizing were not especially high (Szatmari et al., Reference Szatmari, Saigal, Rosenbaum and Campbell1993).
Conclusions
In ELBW survivors, dynamic changes in growth measured by the PI and HC during the first 3 years of postnatal life were indicated by latent change factors (ΔPI, ΔHC) in LDS models. The most vulnerable ELBW infants displayed greater catch-up growth in both of these measures over the first year, and smaller normative declines in the PI over the first 3 years. Changes in the PI indicate relatively short-term responses to available nutrition. In infants born at ELBW, early-life changes in the PI appeared to reflect an immediate survival strategy to maintain weight stores, followed by a dynamic shift after the first year from defensive energy conservation to a strategy that favored physical development. Higher levels of adolescent internalizing behaviors may be a downstream consequence of delayed postnatal diversion of nutritional resources to physical development in ELBW infants. Infants who showed compensatory growth in HC in the first year were relatively more vulnerable, having smaller HC at birth, and higher levels of attentional problems (ADHD) in adolescence. Together, the findings suggest some specificity in the relations between adolescent internalizing behaviors and early proportional growth (reflected in PI change), and between adolescent ADHD problems and early brain development (reflected in HC growth). Importantly, early growth rates in both measures depended on their initial start values, that is, on the degree of growth achieved by the time of birth.
Given these associations, the impact of early nutritional interventions on postnatal growth patterns and brain maturation should be examined in conjunction with long-term psychological functioning. If the timing of the shift favoring neurodevelopment is responsive to early nutritional intervention, it may be possible to minimize or pre-empt potential negative downstream consequences of postnatal undernutrition for later mental health. Additional studies will be required to establish firmer connections between early growth patterns in infants born at ELBW and later mental health.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0954579423000573.
Acknowledgements
This research was supported by grants from the Canadian Institutes of Health Research (CIHR) awarded to Louis A. Schmidt (CIHR Team Grant: TMH-103145; CIHR Operating Grant: IHDCYH-383548). We wish to express our thanks to Lorraine Hoult and Barbara Stoskopf for conducting the childhood and adolescent assessments and to the study participants and their families for their continuing participation in this work.
Competing interest
None.















