Mental health disorders affect approximately 13.4% of children and adolescents, and are associated with increased risk of adverse health outcomes in the short and long term (Polanczyk, Salum, Sugaya, Caye, & Rohde, Reference Polanczyk, Salum, Sugaya, Caye and Rohde2015; Prince et al., Reference Prince, Patel, Saxena, Maj, Maselko, Phillips and Rahman2007). Behavior problems are among the most common mental health complaints in youngsters; they are associated with premature death (von Stumm et al., Reference von Stumm, Deary, Kivimaki, Jokela, Clark and Batty2011) and adult-life illness including depressive, anxiety, bipolar, obsessive-compulsive, and panic disorders (Roza, Hofstra, van der Ende, & Verhulst, Reference Roza, Hofstra, van der Ende and Verhulst2003). Because most behavior disorders develop between 11 and 18 years of age (Patton et al., Reference Patton, Sawyer, Santelli, Ross, Afifi, Allen and M2016), it is essential to understand their determinants at earlier ages.
Infection and the resulting inflammatory response may play an important role in neurobehavioral development and risk of mental illness. Acute infections trigger the immune system to release cytokines and chemokines which help to regulate the immune response. Cytokines can be released directly from neurons and glial cells within the central nervous system or may traverse the blood–brain barrier from the periphery via active transport, diffusion from circumventricular organs, or blood–brain barrier secretion (Jiang, Cowan, Moonah, & Petri, Reference Jiang, Cowan, Moonah and Petri2018). The blood–brain barrier is more permeable during childhood than in adulthood; thus, cytokines are likely to cross it in the presence of an acute childhood infection (Bilbo & Schwarz, Reference Bilbo and Schwarz2012). In the central nervous system, cytokines play a normal role in autocrine and paracrine signaling; notwithstanding, recurrent infections may alter this signaling and affect neurodevelopment in several ways (Bilbo & Schwarz, Reference Bilbo and Schwarz2012; Jiang, Cowan et al., Reference Jiang, Cowan, Moonah and Petri2018). First, an increase in proinflammatory cytokines due to infection can have a direct effect on neurogenesis, neuronal and glial cell migration, proliferation, and differentiation, and synaptic maturation and pruning, all vital processes in neurodevelopment. Second, cytokines can activate the hypothalamic–pituitary axis, increasing glucocorticoid production (Webster & Sternberg, Reference Webster and Sternberg2004), and altering the metabolism of neurotransmitters including norepinephrine and serotonin (Dunn, Reference Dunn2006). Glucocorticoids can directly affect neurodevelopmental processes including myelination and programmed cell death (Huang, Reference Huang2011), while deficits in norepinephrine (Moret & Briley, Reference Moret and Briley2011) and serotonin (Van Praag, Reference Van Praag1982) are commonly observed in patients with depression. Although critical neurodevelopmental events occur in utero and before 3 years of age, there is evidence that substantial synapse growth and myelination continue throughout early to middle childhood; thus, this period constitutes a key window during which infection and inflammation may impact brain development and downstream behavioral outcomes (John, Black, & Nelson, Reference John, Black and Nelson2017).
Exposure to enteropathogens at birth has been related to decreased cognitive performance after 24 months of age (Mal-Ed Network Investigators, 2018). Further, helminthiases (Ezeamama et al., Reference Ezeamama, Friedman, Acosta, Bellinger, Langdon, Manalo and McGarvey2005), schistosomiasis (Nokes et al., Reference Nokes, McGarvey, Shiue, Wu, Wu, Bundy and Olds1999), and malaria (Nankabirwa et al., Reference Nankabirwa, Wandera, Kiwanuka, Staedke, Kamya and Brooker2013) in middle childhood have been related to impaired cognitive development. In addition, infections requiring ambulatory or hospital treatment in infancy and childhood have been associated with subsequent development of severe mental health disorders during childhood and adolescence (Blomström et al., Reference Blomström, Karlsson, Svensson, Frisell, Lee, Dal and Dalman2014; Köhler-Forsberg et al., Reference Köhler-Forsberg, Petersen, Gasse, Mortensen, Dalsgaard, Yolken and Benros2019). The role in behavioral development of recurrent infections at life stages when they are common, including middle childhood, has not been elucidated. Evidence is also scant on the associations between biomarkers of subclinical inflammation, often resulting from recurrent infections, and neurobehavioral outcomes.
The objective of this study was to ascertain whether high incidence of common middle childhood infections was related to the development of internalizing and externalizing behavior problems in adolescence. A secondary aim was to examine the associations between biomarkers of inflammation of any grade, which may result from acute or recurrent infections, and behavior problems.
Method
Study design and population
The study was conducted in the context of the Bogotá School Children Cohort, a longitudinal investigation of health and nutrition in Colombia. Details on the cohort design have been previously reported (Arsenault et al., Reference Arsenault, Mora-Plazas, Forero, Lopez-Arana, Marin, Baylin and Villamor2009; Robinson et al., Reference Robinson, Marin, Oliveros, Mora-Plazas, Richards, Lozoff and Villamor2018). In brief, we recruited 3,202 randomly selected children aged 5–12 years from public primary schools in February 2006. Since most children in the public school system are from low- and middle-income backgrounds, the sample pertains to these groups.
Baseline information
At the time of enrollment, we collected information on child, parental, and household characteristics with the use of a parental self-administered survey. The questionnaire inquired about the child's health habits, maternal education, height, and weight, and household food insecurity and socioeconomic status. Household food insecurity was assessed with a validated Spanish language version of the US Department of Agriculture household food security survey module (Harrison, Stormer, Herman, & Winham, Reference Harrison, Stormer, Herman and Winham2003) and household socioeconomic status was categorized according to the city government's classification.
During the weeks following enrollment, trained research assistants visited participating children at their schools to perform anthropometric measurements. Height was measured without shoes to the nearest 1 mm using a wall-mounted portable Seca 202 stadiometer (Seca, Hanover, MD) and weight was measured in light clothing to the nearest 0.1 kg using Tanita H5301 electronic scales (Tanita, Arlington Heights, IL). At the same visits, a fasting blood sample was obtained through antecubital venipuncture. One aliquot was collected in a tube coated with ethylenediaminetetraacetic acid, and a second one in a metal-free polypropylene tube without anticoagulant for separation of serum. The samples were protected from sunlight and transported in refrigerated coolers on the day of collection to the Colombian National Institute of Health, where they were processed and cryostored for future analyses.
Throughout the academic year following enrollment into the cohort, parents or primary caregivers kept daily records of morbidity episodes using a 7-day pictorial diary that was distributed and returned on a weekly basis. The diaries had drawings depicting children with symptoms including vomiting, diarrhea, fever, cough, and earache/discharge. Caregivers were asked to record the presence of these symptoms daily on check boxes. Diaries have been used to register participants’ symptoms in studies of gastrointestinal and respiratory illness, including randomized controlled trials in which illness is defined according to clinical symptoms (Blanken et al., Reference Blanken, Rovers, Molenaar, Winkler-Seinstra, Meijer, Kimpen and Bont2013; Martin, Fairchok, Stednick, Kuypers, & Englund, Reference Martin, Fairchok, Stednick, Kuypers and Englund2013; Pappas, Hendley, Hayden, & Winther, Reference Pappas, Hendley, Hayden and Winther2008). The use of symptom diaries has been validated in various settings (Stanton et al., Reference Stanton, Clemens, Aziz, Khatun, Ahmed and Khatun1987; Watson, Little, Moore, Warner, & Williamson, Reference Watson, Little, Moore, Warner and Williamson2001), and previous studies indicate that pictorial diaries validly capture incidence of morbidity in low- and middle-income countries (Goldman, Vaughan, & Pebley, Reference Goldman, Vaughan and Pebley1998; Wright et al., Reference Wright, Gundry, Conroy, Wood, Du Preez, Ferro-Luzzi and Potgieter2006).
Follow-up
Between 2011 and 2015 we conducted an in-person follow-up assessment in a random sample of approximately one-third of cohort members (n = 1,139). At this assessment, we ascertained adolescent behavior from the parents’ and child's perspectives with use of the Spanish language versions of the child behavior checklist (CBCL) and the youth self-report (YSR) questionnaire, respectively. The CBCL has been validated for use in children aged 5–18 years (Achenbach & Rescorla, Reference Achenbach and Rescorla2001), has high reliability (Achenbach et al., Reference Achenbach, Bird, Canino, Phares, Gould and Rubio-Stipec1990), and has been utilized in Brazil (Borsa, Reference Borsa2015) and Colombia (Hewitt-Ramírez et al., Reference Hewitt-Ramírez, Gantiva, Vera Maldonado, Cuervo Rodríguez, Hernández Olaya, Juárez and Parada2014). The YSR has been validated for use in adolescents aged 11–18 years (Achenbach & Rescorla, Reference Achenbach and Rescorla2001) and has high reliability (Achenbach et al., Reference Achenbach, Bird, Canino, Phares, Gould and Rubio-Stipec1990). It is also generalizable to Spanish-speaking populations (Ivanova et al., Reference Ivanova, Achenbach, Rescorla, Dumenci, Almqvist, Bilenberg and Verhulst2007) and has been utilized in studies of Chilean (Lozoff, Castillo, Clark, Smith, & Sturza, Reference Lozoff, Castillo, Clark, Smith and Sturza2014), Costa Rican (Corapci, Calatroni, Kaciroti, Jimenez, & Lozoff, Reference Corapci, Calatroni, Kaciroti, Jimenez and Lozoff2010), and Puerto Rican (Achenbach et al., Reference Achenbach, Bird, Canino, Phares, Gould and Rubio-Stipec1990) adolescents. Both instruments consist of 112 statements about behaviors and feelings that the respondents rate as false, sometimes true, or very often true. From responses to these questions, an assessment data manager software (Achenbach System of Empirically Based Assessment, 2010) calculates continuous scores for eight behavior problem subscales: aggressive behavior, rule breaking behavior, anxious/depressed, withdrawn/depressed, somatic complaints, attention problems, social problems, and thought problems. The sum of the anxious/depressed, withdrawn/depressed, and somatic complaints subscale scores comprise the internalizing problems score and the sum of the aggressive and rule breaking behavior subscale scores constitute the externalizing problems score (Bordin et al., Reference Bordin, Rocha, Paula, Teixeira, Achenbach, Rescorla and Silvares2013). The assessment data manager software standardizes scores by age and sex to a reference population derived from data collected periodically in US national surveys (Achenbach System of Empirically Based Assessment, 2010).
The parents or primary caregivers of all children gave written informed consent prior to enrollment in the study and before participation in the follow-up assessment. Youth gave written assent to participate. The Ethics Committee of the National University of Colombia Medical School approved the study protocol. The University of Michigan Institutional Review Board approved the use of data from the study.
Laboratory methods
All analyses took place at the Colombian National Institute of Health. We carried out a complete blood count in whole blood; serum C-reactive protein (CRP) concentration was measured using a turbidimetric immunoassay on an ACS180 analyzer (Bayer Diagnostics, Tarrytown, NY). Plasma ferritin and vitamin B12 were quantified using a competitive chemiluminescent immunoassay in an ADVIA Centaur analyzer (Bayer Diagnostics, Tarrytown, NY).
Data analysis
Outcomes
Primary outcomes were total internalizing and externalizing behavior problem scores per the CBCL and YSR. Secondary outcomes were the individual subscale scores.
Exposures
The exposures of interest were (a) infectious morbidity in middle childhood, (b) white blood cell (WBC) counts at baseline, and (c) serum CRP concentrations at baseline. Infectious morbidity exposures were defined using the parental report of symptoms on the pictorial diaries administered during the first year of follow-up. We defined three syndromes to represent infectious morbidity by combining symptoms reported on the same day in the pictorial diaries: diarrhea with vomiting, cough with fever, and earache or ear discharge with fever. Diarrhea with vomiting has been related to clinically diagnosed episodes of gastrointestinal illness (Arias et al., Reference Arias, Sala, Dominguez, Torner, Ruiz, Martinez and Buesa2010; Rockx et al., Reference Rockx, De Wit, Vennema, Vinje, De Bruin, Van Duynhoven and Koopmans2002; Staat et al., Reference Staat, Azimi, Berke, Roberts, Bernstein, Ward and Matson2002). Cough with fever had a positive predictive value of 83% for laboratory-confirmed influenza infection among children 5–12 years old (Ohmit & Monto, Reference Ohmit and Monto2006), and this case definition has been used to monitor influenza-like illness in Latin America (Gordon et al., Reference Gordon, Ortega, Kuan, Reingold, Saborio, Balmaseda and Harris2009). Cough with fever is also reported in school-age children experiencing the common cold due to a variety of viral and bacterial infections (Pappas et al., Reference Pappas, Hendley, Hayden and Winther2008). Although the diagnosis of acute otitis media requires clinical examination, symptoms including moderate to severe ear pain with fever are indicators of severe illness (Lieberthal et al., Reference Lieberthal, Carroll, Chonmaitree, Ganiats, Hoberman, Jackson and Tunkel2013) and ear drainage is often related to bacterial infection (Chen, Hsieh, Huang, & Chiu, Reference Chen, Hsieh, Huang and Chiu2013). We also considered any fever (alone or in combination with any other symptom) as an unspecific infection surrogate. Because there is not a conventionally accepted categorization of morbidity rates in middle childhood, we used the study population distributions to define morbidity levels as previously done in other populations (Grüber et al., Reference Grüber, Keil, Kulig, Roll, Wahn, Wahn and Study Group2008; Ramette et al., Reference Ramette, Spycher, Wang, Goutaki, Beardsmore and Kuehni2018). Since a majority of children had no episodes, zero rates (“none”) were the reference category for all infectious syndromes. Next, among children with at least one episode, we used quantiles of the distributions. For fever, we divided children with rates >0 into three equal-sized groups (tertiles) (Grüber et al., Reference Grüber, Keil, Kulig, Roll, Wahn, Wahn and Study Group2008), representing “low,” “moderate,” or “high” rates, respectively. For all other syndromes, reduced variability in rates >0 prevented us from using tertiles; thus, we divided the population with non-zero rates as under versus at or above the median rate, to represent “moderate” or “high” rates, respectively. Rates were not considered as continuous variables in the analyses because they were highly overdispersed. WBC is an established biomarker for infection and inflammation in clinical practice; it has been used in pediatric population studies to assess chronic inflammation (Abramson & Melton, Reference Abramson and Melton2000; Adelantado-Renau, Beltran-Valls, Mota, & Moliner-Urdiales, Reference Adelantado-Renau, Beltran-Valls, Mota and Moliner-Urdiales2020). High WBC has been related to cardiometabolic risk factors in this (Gilbert-Diamond, Baylin, Mora-Plazas, & Villamor, Reference Gilbert-Diamond, Baylin, Mora-Plazas and Villamor2012) and other (Lee et al., Reference Lee, Shin, Kim, Shim, Kang and Lee2010; Park, Lee, & Lee, Reference Park, Lee and Lee2017) pediatric surveys, supporting the notion that increased WBC may serve as a low-cost marker of inflammation, which may result from frequent infections. Elevated WBC count was defined as >10,000/mm3. Among children, the normal WBC range is between 5,000 and 10,000. Values above 10,000 are clinically significant (Cleveland Clinic, Reference Cleveland Clinic2018). WBC was also considered as a continuous predictor. CRP is a low-cost, validated, frequently used biomarker to detect systemic inflammation (Abramson & Melton, Reference Abramson and Melton2000; Singh & Newman, Reference Singh and Newman2011; Stolzman & Bement, Reference Stolzman and Bement2012). Elevated CRP was defined as >3.0 mg/L, which has been previously used to define low-grade systemic inflammation in children (Broyles et al., Reference Broyles, Staiano, Drazba, Gupta, Sothern and Katzmarzyk2012; Lande et al., Reference Lande, Pearson, Vermilion, Auinger and Fernandez2008). CRP was also considered as a continuous predictor after logarithmic transformation.
Statistical analysis
Of the 1,139 children in the follow-up assessment, 1,044 (92%) had valid information on the CBCL or the YSR. Twenty-six children with missing morbidity data in middle childhood were excluded, resulting in a final analytic sample of 1,018 children (818 with CBCL and 1,016 with YSR). Compared with cohort participants excluded from analyses, children in the analytic sample had higher rates of diarrhea with vomiting, cough with fever, and any fever, but these differences were not statistically significant (see Table 1 in the Supplementary Material). They were also more likely to be female, were slightly younger, and had better educated mothers.
We compared the continuous distribution of internalizing and externalizing behavior problem scores between categories of each exposure using means and standard deviation, SD. Next, we estimated adjusted mean differences and 95% confidence intervals (CI) with use of linear regression models. Confounders were independent predictors of behavior problems as previously reported in this population (Robinson et al., Reference Robinson, Marin, Oliveros, Mora-Plazas, Richards, Lozoff and Villamor2018). These included child's sex, age, baseline iron deficiency (serum ferritin <15 μg/l in children with CRP ≤10 mg/l), hemoglobin, and low plasma vitamin B12 concentrations, mother's education, household food insecurity with hunger, and low socioeconomic status. Children with CRP >10 mg/l were excluded from multivariable models because iron deficiency per serum ferritin, an adjustment covariate, is undefined in this group. Tests for linear trend were carried out by introducing into the models a variable representing ordinal categories of the exposure as a continuous covariate. Because there were 50 sibling pairs and 1 triplet, all models specified robust variances and accounted for correlations between siblings. Analyses were performed with use of the Statistical Analysis Software version 9.4 (SAS Institute, Cary, NC).
Results
Mean ± SD children's age at baseline was 8.5 ± 1.6 years and 14.7 ± 1.7 years at follow-up; 56.2% were girls (see Table 1 in the Supplementary Material). Median follow-up was 6.1 years (mean ± SD: 6.2 ± 1.0 years, range: 5.2–9.5 years). Children contributed 126,877 days of observation for infectious morbidity during the first follow-up year (median per child, 133; interquartile range, 77, 175). Annual morbidity rates (days per child-year) of diarrhea with vomiting, cough with fever, ear pain/ear discharge with fever, and fever were, respectively, 1.0, 3.2, 0.7, and 5.9. Mean ± SD baseline WBC was 7,137 ± 2,071/mm3 and 6.9% had WBC >10,000/mm3. Mean ± SD CRP was 1.4 ± 2.5 mg/l, 7.7% had CRP >3.0 mg/l and 1.3% had values >10.0 mg/l, a cut point suggestive of an acute infection.
Morbidity in middle childhood and internalizing problems in adolescence
Mean ± SD CBCL and YSR internalizing problems scores were, respectively, 56.8 ± 9.8 and 53.8 ± 9.9. Diarrhea with vomiting and cough with fever rates were positively related to the CBCL internalizing problems score (Table 1, Figure 1). The mean score of children who experienced moderate diarrhea with vomiting (>0 to ≤4.6 days/year) was an adjusted 2.5 (95% CI: 0.1, 4.9; p = .04) units higher than that of children without these symptoms. High cough with fever rates (≥6.6 days/year) were related to an adjusted 3.1 (95% CI: 1.1, 5.2; p = .003) units higher mean CBCL internalizing problems score compared with zero rates. High cough with fever rates were also related to increased CBCL somatic complaints (see Table 2 in the Supplementary Material) and anxious/depressed (see Table 3 in the Supplementary Material) subscale scores. Earache/discharge with fever was positively associated with higher CBCL somatic complaints subscale scores (see Table 2 in the Supplementary Material), whereas high fever rates (≥13.5 days/year) were related to increased CBCL anxious/depressed subscale scores (see Table 3 in the Supplementary Material). There were no associations with the withdrawn/depressed subscale (see Table 4 in the Supplementary Material).
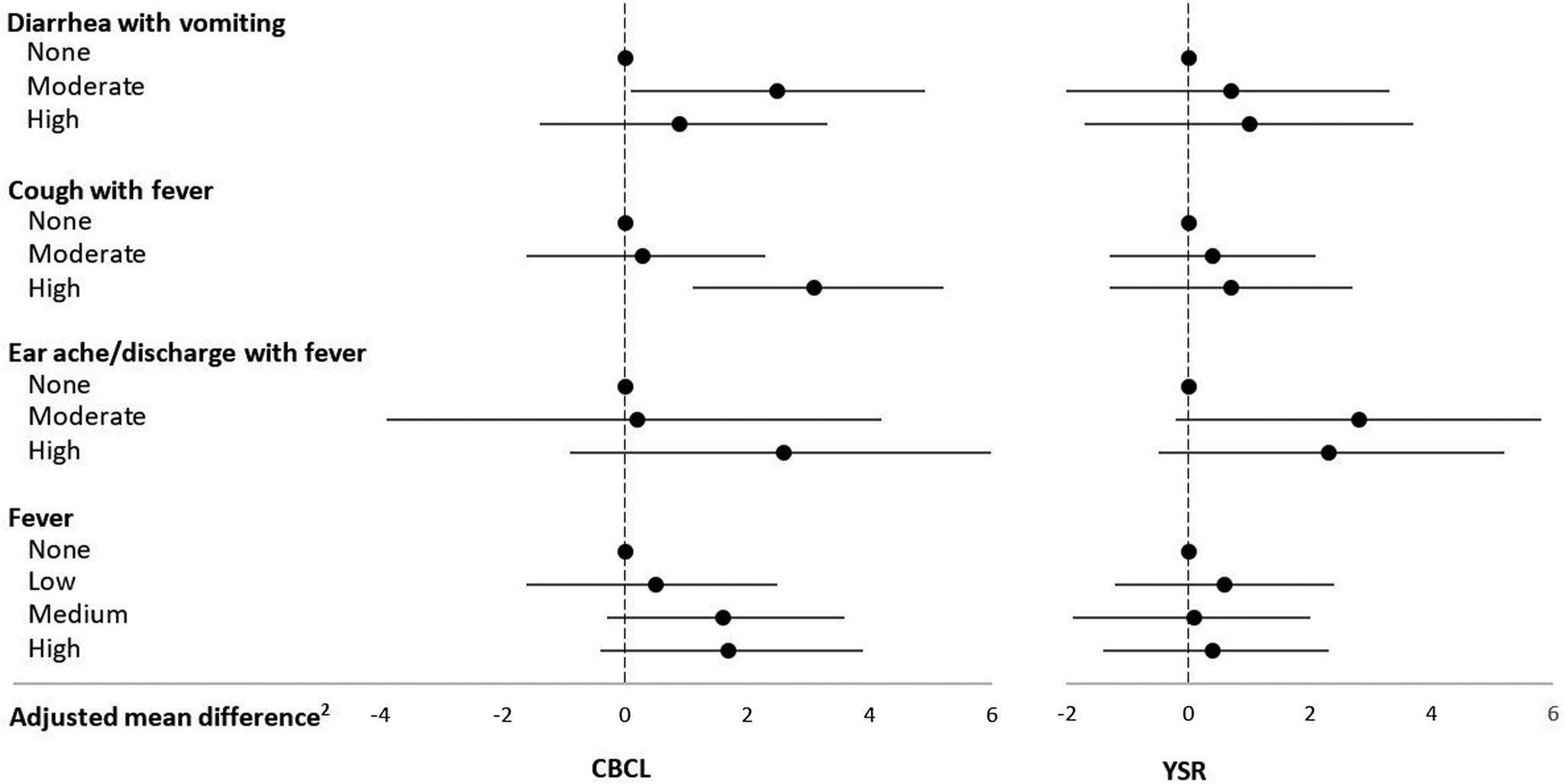
Figure 1. Infectious morbidity symptoms (days per year)1 in middle childhood and total internalizing problems in adolescence among schoolchildren from Bogotá, Colombia. CBCL: child behavior checklist (parent report); YSR: youth self-report. 1Moderate and high number of days per year correspond to values < versus ≥ the median for children with rates >0. Medians (days per year) are 4.6, 6.6, and 3.9 for diarrhea with vomiting, cough with fever, and earache/discharge with fever, respectively. For fever, low, moderate, and high are tertiles of the distribution among children with rates >0 and correspond to cut points (days per year) 4.7 and 13.5, respectively. 2Horizontal lines represent 95% confidence intervals. Estimates are from linear regression models adjusted for child's sex, age, iron deficiency, anemia, and low vitamin B12 at baseline, mother's education, household food insecurity with hunger, and low socioeconomic status. Robust estimates of variance were used in all models to account for correlations between siblings.
Table 1. Infectious morbidity symptoms in middle childhood and total internalizing problems in adolescence among schoolchildren from Bogotá, Colombia
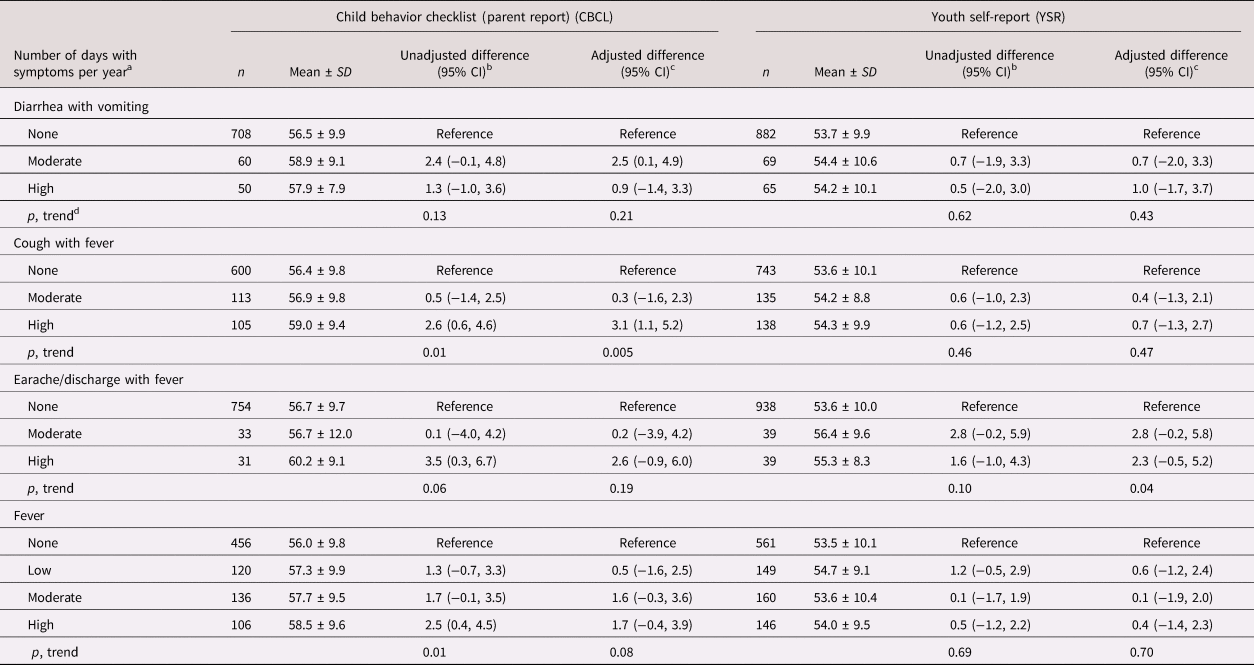
a Moderate and high number of days per year correspond to values < versus ≥ the median for children with rates >0. Medians (days per year) are 4.6, 6.6, and 3.9 for diarrhea with vomiting, cough with fever, and earache/discharge with fever, respectively. For fever, low, moderate, and high are tertiles of the distribution among children with rates >0 and correspond to cut points (days per year) 4.7 and 13.5, respectively.
b From linear regression models with total internalizing behavior problems score as the continuous outcome and indicator variables for each combination of symptoms as predictors. Robust estimates of variance were used in all models to account for correlations between siblings.
c From linear regression models adjusted for child's sex, age, iron deficiency, anemia, and low vitamin B12 at baseline, mother's education, household food insecurity with hunger, and low socioeconomic status. Robust estimates of variance were used in all models to account for correlations between siblings.
d Wald test for a variable representing the median value of each ordinal category introduced into the linear regression model as a continuous predictor.
Morbidity in middle childhood and externalizing problems in adolescence
Mean ± SD CBCL and YSR externalizing problems scores were, respectively, 55.6 ± 9.2 and 52.6 ± 9.6. Earache/discharge with fever was associated with increased YSR externalizing behavior problems score (Table 2, Figure 2). Other morbidity rates were not associated with externalizing problems per the CBCL or the YSR overall or with the rule-breaking (see Table 5 in the Supplementary Material) or aggressive behavior (see Table 6 in the Supplementary Material) subscales.
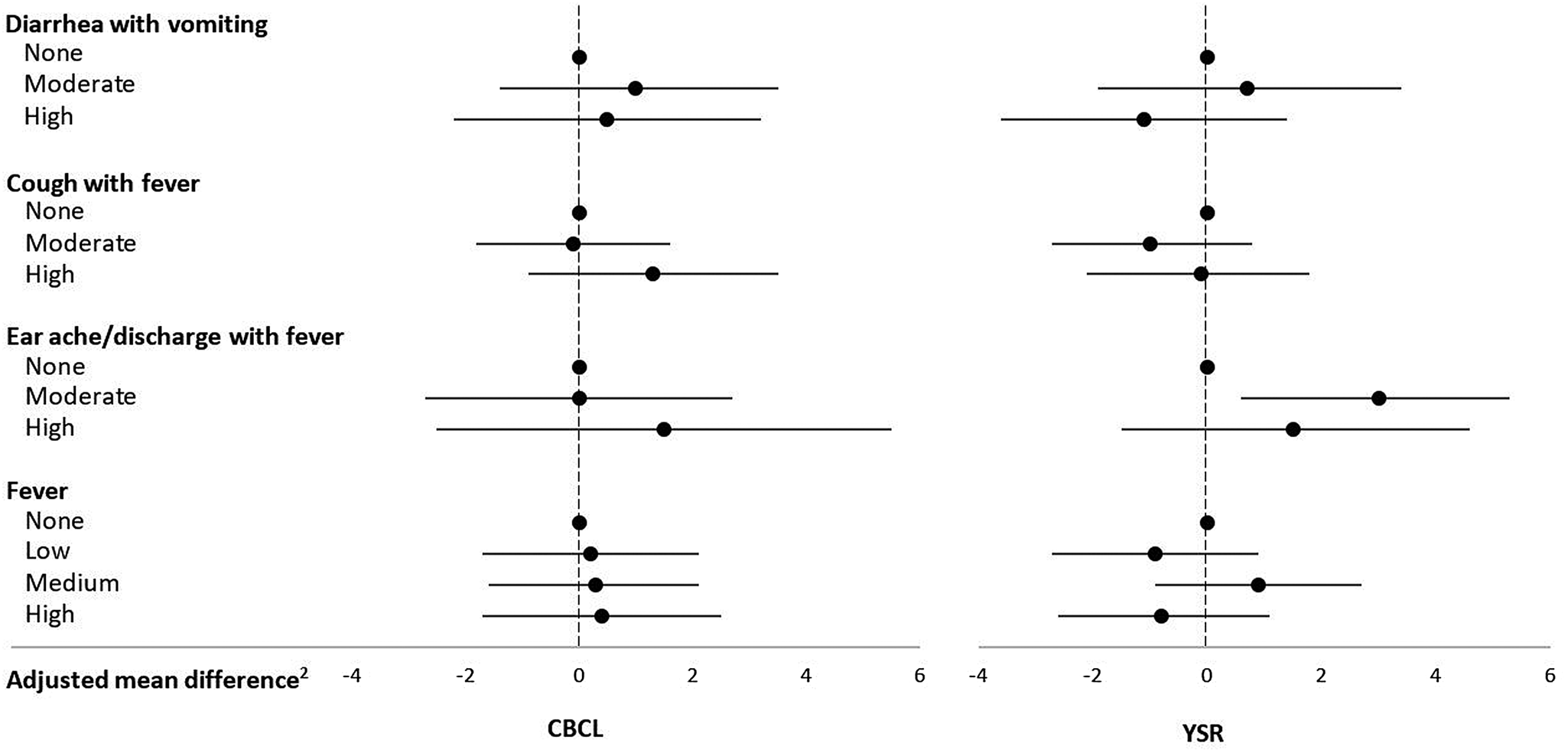
Figure 2. Infectious morbidity symptoms (days per year)1 in middle childhood and total externalizing problems in adolescence among schoolchildren from Bogotá, Colombia. CBCL: child behavior checklist (parent report); YSR: youth self-report. 1Moderate and high number of days per year correspond to values < versus ≥ the median for children with rates >0. Medians (days per year) are 4.6, 6.6, and 3.9 for diarrhea with vomiting, cough with fever, and earache/discharge with fever, respectively. For fever, low, moderate, and high are tertiles of the distribution among children with rates >0 and correspond to cut points (days per year) 4.7 and 13.5, respectively. 2Horizontal lines represent 95% confidence intervals. Estimates are from linear regression models adjusted for child's sex, age, iron deficiency, anemia, and low vitamin B12 at baseline, mother's education, household food insecurity with hunger, and low socioeconomic status. Robust estimates of variance were used in all models to account for correlations between siblings.
Table 2. Infectious morbidity symptoms in middle childhood and total externalizing problems in adolescence among schoolchildren from Bogotá, Colombia
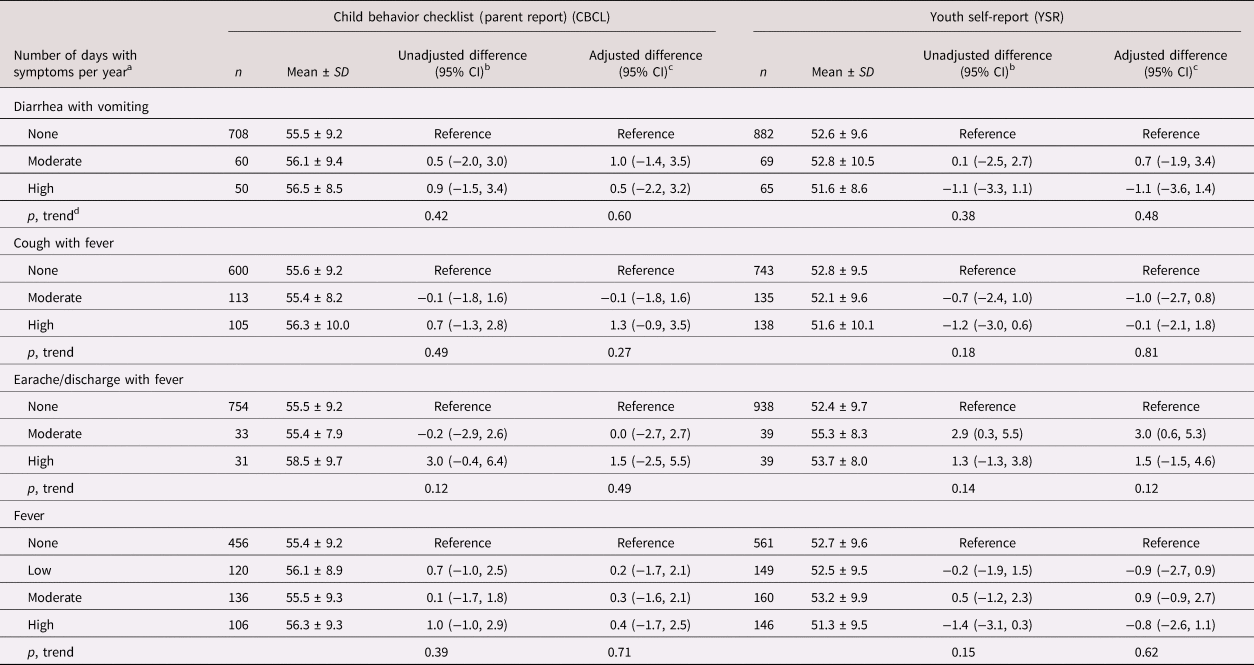
a Moderate and high number of days per year correspond to values < versus ≥ the median for children with rates >0. Medians (days per year) are 4.6, 6.6, and 3.9 for diarrhea with vomiting, cough with fever, and earache/discharge with fever, respectively. For fever, low, moderate, and high are tertiles of the distribution among children with rates >0 and correspond to cut points (days per year) 4.7 and 13.5, respectively.
b From linear regression models with total externalizing behavior problems score as the continuous outcome and indicator variables for each combination of symptoms as predictors. Robust estimates of variance were used in all models to account for correlations between siblings.
c From linear regression models adjusted for child's sex, age, iron deficiency, anemia, and low vitamin B12 at baseline, mother's education, household food insecurity with hunger, and low socioeconomic status. Robust estimates of variance were used in all models to account for correlations between siblings.
d Wald test for a variable representing the median value of each ordinal category introduced into the linear regression model as a continuous predictor.
Inflammatory biomarkers in middle childhood and internalizing problems in adolescence
WBC count was positively associated with internalizing problem scores (Table 3, Figure 3). Compared with children whose WBC counts were ≤10,000/mm3, those with WBC >10,000/mm3 had an adjusted 2.9 (95% CI: 0.3, 5.5, p = .04) and 2.9 (95% CI: 0.1, 5.6, p = .05) units higher CBCL and YSR internalizing behavior score, respectively. Although WBC count was not associated with the somatic complaints (see Table 7 in the Supplementary Material) or the anxious/depressed (see Table 8 in the Supplementary Material) subscale scores, WBC counts >10,000/mm3 were positively related to YSR withdrawn/depressed scores (see Table 9 in the Supplementary Material). CRP plasma concentrations were not associated with internalizing problems scores (Table 3) or its subscales (see Tables 7–9 in the Supplementary Material).

Figure 3. Inflammatory biomarkers in middle childhood and total internalizing problems in adolescence among schoolchildren from Bogotá, Colombia. CBCL: child behavior checklist (parent report); YSR: youth self-report. 1Horizontal lines represent 95% confidence intervals. Estimates are from linear regression models adjusted for child's sex, age, iron deficiency, anemia, and low vitamin B12 at baseline, mother's education, household food insecurity with hunger, and low socioeconomic status. Robust estimates of variance were used in all models to account for correlations between siblings.
Table 3. Inflammatory biomarkers in middle childhood and total internalizing problems in adolescence among schoolchildren from Bogotá, Colombia

a From linear regression models with total internalizing behavior problems score as the continuous outcome and indicator variables for each inflammatory biomarker as predictors. Robust estimates of variance were used in all models to account for correlations between siblings.
b From linear regression models adjusted for child's sex, age, and iron deficiency at baseline, household food insecurity with hunger, and mother's education. Robust estimates of variance were used in all models to account for correlations between siblings.
c Wald test.
Inflammatory biomarkers in middle childhood and externalizing problems in adolescence
Neither WBC counts nor CRP plasma concentrations were associated with total externalizing problems in adolescence (Table 4, Figure 4) or its subscales (see Tables 10 and 11 in the Supplementary Material).
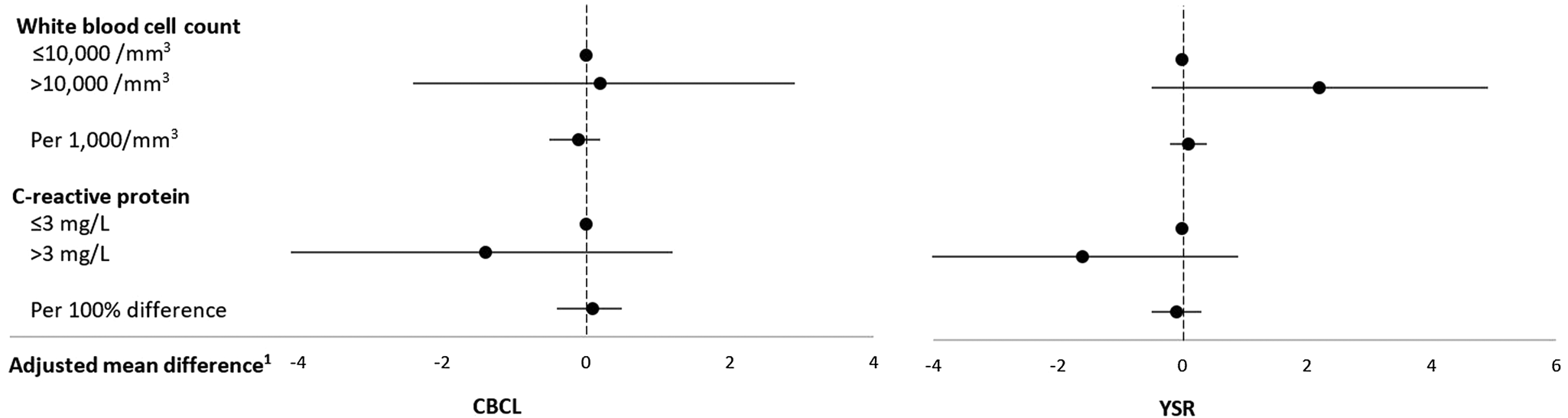
Figure 4. Inflammatory biomarkers in middle childhood and total externalizing problems in adolescence among schoolchildren from Bogotá, Colombia. CBCL: child behavior checklist (parent report); YSR: youth self-report. 1Horizontal lines represent 95% confidence intervals. Estimates are from linear regression models adjusted for child's sex, age, iron deficiency, anemia, and low vitamin B12 at baseline, mother's education, household food insecurity with hunger, and low socioeconomic status. Robust estimates of variance were used in all models to account for correlations between siblings.
Table 4. Inflammatory biomarkers in middle childhood and total externalizing problems in adolescence among schoolchildren from Bogotá, Colombia

a From linear regression models with total externalizing behavior problems score as the continuous outcome and indicator variables for each inflammatory biomarker as predictors. Robust estimates of variance were used in all models to account for correlations between siblings.
b From linear regression models adjusted for child's sex, age, iron deficiency, anemia, and low vitamin B12 at baseline, low socioeconomic status, household food insecurity with hunger, and mother's education. Robust estimates of variance were used in all models to account for correlations between siblings.
c Wald test.
Discussion
In this longitudinal investigation of low- and middle-income Colombian school children, gastrointestinal and respiratory infectious morbidity in middle childhood was associated with internalizing behavior problems in adolescence. The association with respiratory morbidity, including cough with fever and otitis media, was driven by associations with the somatic complaints subscale, whereas fever of any origin was related to increased anxious/depressed scores. Elevated WBC count, a marker of inflammation, was also associated with internalizing behavior problems, through the withdrawn/depressed subscale.
Previous studies reported associations between infectious morbidity in childhood and neurocognitive and behavioral development or psychiatric illness later in life. A population-based cohort study in Denmark found that postnatal infections requiring outpatient or hospital treatment were associated with subsequent risk of severe mental disorders (Köhler-Forsberg et al., Reference Köhler-Forsberg, Petersen, Gasse, Mortensen, Dalsgaard, Yolken and Benros2019), whereas childhood infection-related hospital admission in Sweden was related to risk of nonaffective psychoses in adolescence (Blomström et al., Reference Blomström, Karlsson, Svensson, Frisell, Lee, Dal and Dalman2014). Inflammatory biomarkers have also been related to mental health outcomes in previous studies. For instance, in the Avon longitudinal study of parents and children, interleukin (IL)-6 at age 9 years was associated with depression and psychosis at age 18 years (Khandaker, Pearson, Zammit, Lewis, & Jones, Reference Khandaker, Pearson, Zammit, Lewis and Jones2014) and hypomanic symptoms at age 22 years (Hayes et al., Reference Hayes, Khandaker, Anderson, Mackay, Zammit, Lewis and Osborn2017) in a dose–response manner. CRP and IL-6 in adulthood also predicted risk of depression in Denmark (Wium-Andersen, Ørsted, Nielsen, & Nordestgaard, Reference Wium-Andersen, Ørsted, Nielsen and Nordestgaard2013) and Britain (Gimeno et al., Reference Gimeno, Kivimäki, Brunner, Elovainio, De Vogli, Steptoe and Ferrie2009). Our study extends previous findings by focusing on common middle childhood infections, including respiratory and gastrointestinal illness, which may not be severe enough to warrant treatment. The association of high WBC with internalizing behavior problems is a novel finding. Because WBC is often elevated in the presence of infection, the results are internally consistent.
There are different possible explanations for an association between pediatric infectious morbidity and neurobehavioral development. First, high infection rates during middle childhood resulting from increased exposure or susceptibility to infectious agents could lead to a sustained subclinical inflammatory response. Chronic inflammation could alter brain development through several pathways. Pro-inflammatory signals produced by the immune system can disrupt and increase the permeability of the blood–brain barrier and induce crossing of cells and cytokines that may directly interact with neuronal projections, potentially affecting brain function (Banks & Erickson, Reference Banks and Erickson2010). Pro-inflammatory cytokines might also induce microglia activation, which affects brain development through impaired neurogenesis and dendritic arborization, neurotransmitter dysregulation, and glial proliferation (Hagberg & Carina, Reference Hagberg and Carina2005). Furthermore, pro-inflammatory cytokines may alter the expression of glutamate receptors through the kynurenine pathway (John et al., Reference John, Black and Nelson2017), and activate the hypothalamus–pituitary–adrenal axis, resulting in increased cortisol production and glucocorticoid resistance (Ratnayake, Quinn, Walker, & Dickinson, Reference Ratnayake, Quinn, Walker and Dickinson2013). Nitric oxide, which is produced by macrophages in response to infectious pathogens, might affect neuronal proliferation, differentiation, and synaptogenesis (Banks & Erickson, Reference Banks and Erickson2010; Gibbs, Reference Gibbs2003; Tripathi, Reference Tripathi2007). These pathways have been involved in the development of mood, anxiety, and psychotic disorders (Nawa & Takei, Reference Nawa and Takei2006; Pariante & Lightman, Reference Pariante and Lightman2008; Tsapakis & Travis, Reference Tsapakis and Travis2002). Second, we noted that the associations between infectious morbidity and internalizing behavior problems were more consistent for the parental report than the self-report, and some of these associations were largely explained through the somatic complaints subscale. It is plausible that parents of children with a high infection burden perceive them as more vulnerable than they may be and induce internalization behaviors (De Ocampo, Macias, Saylor, & Katikaneni, Reference De Ocampo, Macias, Saylor and Katikaneni2003). Third, the somatic complaints subscale in the CBCL comprises questions on physical symptoms including constipation, dizziness, tiredness, aches, nausea, cramps, and vomiting (Read et al., Reference Read, Settipani, Peterman, Kendall, Compton, Piacentini and March2015). If the morbidity burden observed during 1 year in middle childhood tracked into adolescence, increased somatic complaints scores could simply reflect an expression of symptoms from frequent illness. Nonetheless, previous research suggests that high parent-reported somatic complaints scores are indicative of somaticizing behavior rather than pain experiences from illness (Campo & Fritsch, Reference Campo and Fritsch1994; Eisman, Fogel, Lazarovich, & Pustilnik, Reference Eisman, Fogel, Lazarovich and Pustilnik2007); thus, somatic complaints may have a psychological rather than a physical basis.
This study has several strengths. The longitudinal design minimizes the possibility of reverse causation bias. Prospective collection of exposure and outcome information precludes misclassification due to recall bias. The use of two complementary scales enhances the validity of outcome assessment since some behaviors may be more or less likely to be reported by adolescents compared to their parents. Finally, the consistency of associations with self-reported morbidity and objectively determined WBC increases the internal validity of the study.
Some limitations are also worth noting. First, reverse causation cannot be completely ruled out if behavior problems were already present in middle childhood; we did not have an opportunity to ascertain them at baseline. Nonetheless, many of these problems only become manifest during adolescence. Second, the analytic sample differed from the group of children excluded from the analyses with respect to the distribution of exposure and some predictors of outcome status; this could lead to selection bias. Third, a single measure of WBC or CRP may not represent chronic subclinical inflammation since their values can increase acutely in response to an infectious episode and may be subject to large within-person variation. Fourth, we lacked information on the etiologic agents of infectious morbidity; this could have provided mechanistic clues. We also lacked information on whether the episodes required treatment. Fifth, residual confounding by unmeasured variables including adverse childhood experiences cannot be discarded. Adverse childhood experiences including foster care and adoption have been related both to gastrointestinal symptoms and anxiety in children and adolescents (Callaghan et al., Reference Callaghan, Fields, Gee, Gabard-Durnam, Caldera, Humphreys and Tottenham2020). Sixth, because there are no agreed-upon categorizations of infectious morbidity in middle childhood, we used distribution-driven definitions of rate cut points and levels and this may limit the comparability of results with other populations. Nevertheless, some of the cut points for high rates that resulted from categorizing them into quantiles seem consistent with those reported in other studies. For example, German schoolchildren had a mean 1.3 common cold episodes per year, each lasting about one week (Grüber et al., Reference Grüber, Keil, Kulig, Roll, Wahn, Wahn and Study Group2008); the definition of high rates of cough with fever in our study (≥7 days/year) would approximately correspond to being above the mean in the German study. Finally, Type I error may be enhanced by analyzing a large number of outcomes; thus, we cannot rule out chance as an explanation for the associations observed.
In conclusion, gastrointestinal and respiratory morbidity and high WBC in middle childhood are associated with internalizing behavior problems in adolescence. Whether decreasing the burden of common infections results in improved neurobehavioral outcomes warrants further investigation.
Supplementary Material
The supplementary material for this article can be found at https://doi.org/10.1017/S0954579421000675
Author Contributions
Rachael J. Beer and Kallisse R. Dent contributed equally to this work.
Funding Statement
This work was supported by the ASISA Research Fund at the University of Michigan.
Conflicts of Interest
None.











