Introduction
Major depressive disorder (MDD) is a debilitating and often chronic disease. By 2020, it is projected to be the second leading cause of disability based on disability-adjusted life-years (DALYs).Reference Murray and Lopez1 In addition, MDD is considered one of the primary causes of disease burden in developed nations, as it is associated with increases in both healthcare service utilization and in public health costs.Reference Murray and Lopez2, Reference Greenberg, Kessler and Birnbaum3
Although a range of antidepressant medications are available for the treatment of MDD, nearly two-thirds of patients do not benefit adequately from an initial course of pharmacotherapy, and continue to be symptomatic and functionally impaired.Reference Sackeim4, Reference Rush, Thase and Dubé5 Current practice guideline recommendations in the setting of initial treatment resistance involve sequential, empirical attempts usually involving progressively more complex forms of medication therapy. For example, medication switches within or between classes; antidepressant combinations; or adjunctive therapy with lithium, thyroid hormone, mood stabilizers, or second-generation antipsychotics are commonly recommended.Reference Janicak, Marder and Pavuluri6 Most of these clinically accepted approaches involve strategies that have not received specific regulatory approval for use in the setting of initial pharmacoresistance. Further complicating clinical management is evidence that the likelihood of achieving remission progressively diminishes with each failed treatment attempt. The Sequenced Treatment Alternatives to Relieve Depression (STAR*D) Study demonstrated that despite best efforts, approximately one-third of patients will remain refractory to pharmacotherapy following four sequential courses of medication at an adequate dose and duration.Reference Fava, Rush and Trivedi7–Reference Rush, Trivedi and Wisniewski14
Transcranial magnetic stimulation (TMS) is a safe and effective treatment option, specifically for patients with MDD who fail to benefit from initial pharmacotherapy.Reference Janicak, O'Reardon and Sampson15–Reference Demitrack and Thase20 The evidence from these randomized controlled trials is recently supported by two large, naturalistic observational studies of TMS in clinical practice settings.Reference Carpenter, Janicak and Aaronson21, Reference Connolly, Helmer and Cristancho22 TMS uses pulsed, MRI-strength magnetic fields to induce electrical currents in the cortex of the brain. These induced currents directly cause neuronal depolarization in local targeted brain regions and indirectly affect more distant regions that are anatomically connected. The result is therapeutically beneficial modulation of the neural circuitry implicated in the pathophysiology of depression.Reference Shafi, Westover and Fox23, Reference Pascual-Leone, Tormos and Keenan24
By design, clinical trials of antidepressant treatments focus on establishing efficacy for specific, symptomatic measures of change, usually involving well-validated clinician and patient-rated illness scales. Recent work focuses attention on the observation that despite clear evidence of symptomatic benefit, many patients experience substantial levels of social and occupational impairment, comparable to the level of disability seen in other serious, chronic medical conditions.Reference Wells, Stewart and Hays25 Further, this impairment in function and quality of life (QOL) tends to persist even after symptomatic remission.Reference Wells, Stewart and Hays25–Reference Kennedy, Foy and Sherazi27 Thus, outcome measures of QOL are increasingly used in clinical trials to assess well-being across several domains of psychosocial functionality.Reference Fairclough28–Reference Judd, Akiskal and Zeller30 This is important since disease-specific measures of illness severity may not accurately describe the overall benefit of a treatment.Reference Trivedi, Rush and Wisniewski31 For example, while medication may improve depressive symptoms, it may also induce unpleasant side effects that impair the patient's perceived QOL.Reference Wisniewski, Rush and Bryan32 Further, McCall etal. Reference McCall, Rosenquist and Kimball33 reported that maximal improvement in QOL may lag weeks or months behind maximum symptom improvement. Thus, a patient's perception of health and well-being does not always correlate with improvement in symptoms alone, but results from a complex interaction between various disease- and treatment-specific contextual factors. Understanding QOL and functional outcomes is critically important in predicting long-term treatment success. For example, psychosocial impairment is associated with a decreased probability of full recovery from a major depressive episode (MDE).Reference Solomon, Leon and Coryell34
Patient-reported measures of QOL, such as the Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36) and the EuroQol 5-Dimensions (EQ-5D), are well-validated and assess functional status and life quality in various health-related, social, and occupational domains.Reference Ware and Sherbourne35, Reference Brooks36 Previous studies found that these instruments are sensitive to change and correlate with clinical improvements in depression.Reference Mann, Gilbody and Richards37
In this multisite, naturalistic, observational study, we examined the effect of TMS on these self-report measures of QOL and functional status in patients with pharmacoresistant MDD. We also examined the relationship between changes in these outcomes with changes in depressive symptoms. Because previous studies found that TMS is devoid of systemic adverse effects and has a low incidence of discontinuation, we hypothesized that clinically meaningful changes in patient-reported QOL outcomes could occur early in treatment.
Methods
Study overview and study population
A complete description of the study design, methods, and patient disposition are reported elsewhere.Reference Carpenter, Janicak and Aaronson21 Briefly, this was a naturalistic observational study conducted at 42 clinical practice locations in the United States: 32 (76%) private clinical practices, 7 (17%) academic medical centers, and 3 (7%) non-academic institutions. Patients were eligible to participate and were considered evaluable for the study data analysis if (1) their primary clinical diagnosis was a MDE [single or recurrent without psychotic features, consistent with Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) criteria]; (2) they did not have medical conditions that precluded the safe use of TMS therapy; (3) they had not received past treatment with TMS for depression; (4) they met standardized criteria for failure to receive clinical benefit from antidepressant medication treatment in the current illness episode; (5) they had a baseline and at least one post-baseline rating; (6) their attending psychiatrist determined that TMS represented the most appropriate clinical treatment option; and (7) the attending psychiatrist initiated treatment using the currently labeled TMS parameters. The methods and instruments used to assess symptomatic change in depression and the outcomes of these measures across acute treatment with TMS in routine clinical practice were previously reported.Reference Carpenter, Janicak and Aaronson21 The purpose of the current report is to provide a comprehensive description of additional patient-reported QOL and functional status outcome measures obtained during acute treatment in the evaluable study population. The number of patients who met these a priori–defined evaluable criteria at baseline was 307, and 286 provided at least 1 post-baseline observation for either the SF-36 or EQ-5D assessments.
There was no limit on the number of lifetime antidepressant treatment failures in study participants. Treatment resistance was determined with the Antidepressant Treatment Record (ATR; Neuronetics, Inc., Malvern, PA, USA). The ATR is a clinician-administered antidepressant treatment history inventory adapted from and validated against the research version of the Antidepressant Treatment History Form (ATHF).Reference Sackeim4 This naturalistic study design permitted patients to continue concurrent psychiatric medications during treatment with TMS if directed by their prescribing psychiatrist. Decisions to administer TMS adjunctively reflected a determination that these agents could not be safely discontinued [300 of 307 (97.7%) of patients were taking one or more psychotropic medications at study baseline].
Institutional review board (IRB) approval was obtained at all participating sites. After a complete description of the study, written informed consent was obtained from all subjects prior to any study-related procedures.
TMS device and clinical treatment parameters
All treatments were delivered using the NeuroStar TMS Therapy system (Neuronetics, Inc.). Motor threshold (MT) was determined over the left hemisphere at the initial session and used for determination of treatment intensity. An iterative, automated software-based mathematical algorithm (MT Assist, Neuronetics, Inc.) is integrated with this system for use in MT determination. External coordinates for placement of the coil over the treatment location are calculated by the device for a site 5 cm anterior from the MT location, along a left superior oblique plane. The standard treatment protocol described in the product user manual specifies stimulation at 120% of MT, pulse frequency of 10 pulses per second, and a cycle of 4 seconds on (active stimulation) and 26 seconds off (no stimulation). This system provides default parameters that generate 75 stimulation cycles, resulting in 3000 pulses per treatment session. While all clinicians initiated treatment with left-sided, high frequency stimulation, the treatment protocol could be modified for tolerability or logistical reasons, or as a consequence of clinician-determined variation in practice technique. Two hundred eighty (91.2%) patients received standard labeled treatment over the left dorsolateral prefrontal cortex only. The proportion of patients treated with variations in technique beyond the standard labeled treatment protocol was too few to provide meaningful comment on any potential differences in clinical outcome for this subset of patients (data not shown).
Functional status and quality of life outcome measures
Patient-reported outcome measures of functional status and QOL were obtained at baseline prior to the initial TMS treatment, and again at the end of acute treatment.
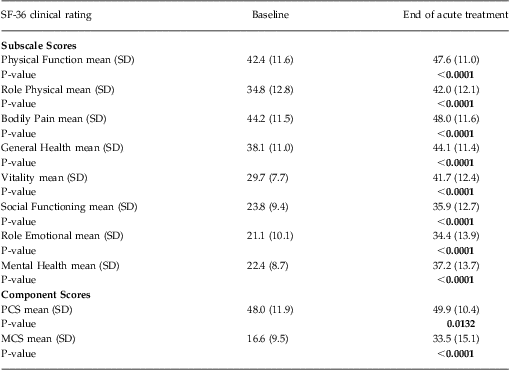
Functional status was ascertained using the Medical Outcome Study Short-Form Health Survey, (SF-36) version 1. This well-validated, self-administered questionnaire measures functional health status and is a criterion standard for health-related QOL.Reference Ware, Kosinski and Gandek38 It contains 8 subscales that measure physical and role functioning, bodily pain, general health, vitality, social and role functioning, and mental health. Higher scores on each scale indicate better functioning in the specific domain. The 8 subscales form 2 distinct higher-ordered clusters of physical and mental health. The physical component summary (PCS) score and mental component summary (MCS) score integrate information from all 8 of the domain subscales. The PCS and MCS are adjusted by the population mean and standard deviation to produce norm-based scores with a common mean of 50 and standard deviation of 10. Thus, any score < 50 represents a decrement from “normal” health and functioning.
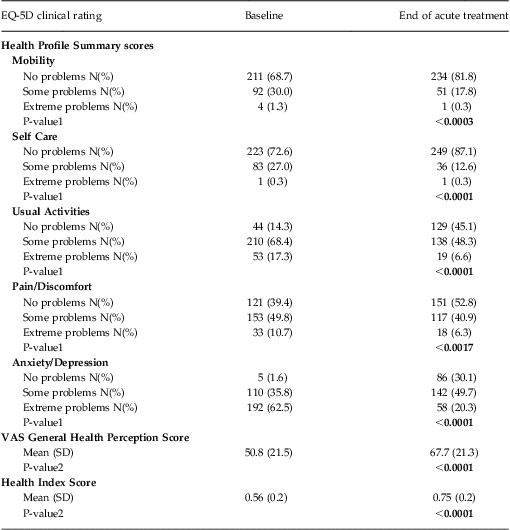
Patient-reported QOL was characterized using the EuroQol 5-Dimension Questionnaire (EQ-5D). This well-validated, self-administered questionnaire measures functional health status and is a criterion standard for health-related QOL.39 It contains 5 dimensions measuring the degree of impairment in the domains of mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. Each dimension has 3 levels: no problems, some problems, or extreme problems; the patient is asked to indicate his or her health status in the specific domain. The EQ-5D also uses a visual analog scale to assess a patient's General Health Perception, with endpoints anchored at the “best imaginable health state” and the “worst imaginable health state.”
The EQ-5D also computes a composite Health Index score derived from the scoring obtained on the 5 health dimensions, and incorporating the U.S. population-based preference weights as published on the Agency for Healthcare Research and Quality Web site For the U.S. general population40, the possible EQ-5D Health Index scores range from –0.11 to 1.0 on a scale where 0.0 = death and 1.0 = perfect health (note that the EQ-5D is capable of characterizing outcomes that may be perceived by the patient as resulting in a QOL measure of “worse than death”).Reference Macran and Kind41
Statistical analysis
For continuous variables, within-group testing comparing the baseline and the end of acute treatment observation time points was performed using the Student's t-test for normally distributed data and the Wilcoxon Signed Rank test for non-normally distributed data. Normality testing was evaluated using the Shapiro–Wilk statistic. Categorical variables were tested using a chi-square analysis. All tests were two-sided, at the 5% level of statistical significance. Results are reported at the 2 observation time points of baseline and end of acute treatment. Because the overall discontinuation rate was less than 15%, nearly all patients who entered acute treatment provided information at both observations. Therefore, an observed case analysis (including all available data) on the intent-to-treat population was used as the primary analysis set.
A number of secondary analyses were also conducted. First, we compared the relationship between the magnitude of change in functional status or QOL to the magnitude of the change in depression symptom scores to assess the level of impairment in those patients who received the most complete symptomatic benefit from treatment. To accomplish this, between-group comparisons were performed on end of acute treatment clinician-rated remission versus non-remission population subgroups. For purposes of this analysis, clinician-rated remission was defined using the Clinician Global Impressions—Severity of Illness Scale (CGI-S) as in the original depression symptom efficacy outcomes reported from this study.Reference Carpenter, Janicak and Aaronson21 In that analysis, remission was defined as an end of acute treatment CGI-S score of 1 or 2.
Second, to determine whether the improvement in functional status or QOL was evident across the full range of symptom distress, we examined the baseline to end of acute treatment change in the two SF-36 component scores (PCS and MCS) and in the EQ-5D Health Index score in subgroups stratified based on their baseline Patient Health Questionnaire (PHQ-9) total score (see Figure 1 for definition of category groupings). We chose the PHQ-9 as the method of baseline symptom stratification in this analysis because it has the least variance among the measures, and published literature exists defining these symptom severity groupings for the PHQ-9.Reference Katzelnick, Duffy and Chung42 Finally, because there are several clinical variables that may influence a patient's response to treatment, we performed a secondary analysis to determine potential predictors of improvement in functional status and QOL outcome with TMS treatment.Reference Cui, Faries, Gelwicks, Novick and Liu43, Reference Hardeveld, Spijker, De Graaf, Nolen and Beekman44 The candidate pre-treatment variables and their method of stratification included the following: gender (M/F), age (age ≤ 55 years vs > 55 years), employment status (employed full time, part-time, or unemployed), salary (< $50,000 vs ≥ $50,000), the presence of a secondary anxiety disorder diagnosis (Y/N), ATR status at baseline (baseline ATR ≤ 1 vs ≥ 2), and depression symptom severity at baseline (as a continuous variable measured by the CGI-S, PHQ-9, and IDS-SR). We screened these candidate variables following a methodological framework described in our previous report.Reference Carpenter, Janicak and Aaronson21 In that method, we used an analysis of variance model to explore the candidate predictors, with the criterion that a potentially significant moderating variable should demonstrate a main effect at a P < 0.10, and its influence on outcome should be evident at this level of statistical strength across the three main composite indices of functional status and QOL improvement (ie, the SF-36 component scores, PCS and MCS, and the EQ-5D Health Index score).

Figure 1 SF-36 Mental Component and Physical Component Scores, EQ-5D Health Index Score: outcome stratified by baseline patient-reported depression symptom severity. Notes: All data were computed using an observed case analysis. Mild Depression = PHQ-9 total score < 10; Moderate Depression = PHQ-9 total score 11 to 15; Moderately Severe Depression = PHQ-9 total score 16 to 20; Severe Depression = PHQ-9 total score > 20. Within-group comparison of change from baseline to end of treatment performed using Student's T-Test: * = P < 0.01, ** = P < 0.001, *** = P < 0.0001. Overall analysis of variance model showed no statistically significant differences in the change from baseline score between the PHQ-9 Depression Severity categories.
Results
Demographic and clinical characteristics
Demographic and clinical characteristics of the study population are shown in Table 1. A recurrent course of illness was reported in 93% of patients, and 44% had previously been hospitalized for depression. A significant level of treatment resistance was present, with over half of the population meeting ATR criteria for failure to benefit from two or more antidepressant trials of adequate dose and duration during the current episode. Baseline measures of functional status and QOL were significantly impaired. For example, the SF-36 MCS score at baseline was more than 2 standard deviations below the norm-based reference population standardized mean score.Reference Ware and Kosinski45 Baseline measures of symptom severity were consistent with a moderate to severe degree of depression prior to treatment.
Table 1 Demographic and clinical characteristics of study population (N = 307)

Patient-reported functional status and QOL outcomes
SF-36—subscale scores and component scores
Subscale scores on the SF-36 at baseline and at the end of acute TMS treatment are summarized in Table 2. The largest treatment effects were observed on those subscale scores associated with improvements in mental health and social functioning (ie, vitality, social functioning, role emotional, and mental health subscales). A correspondingly larger improvement was observed in the MCS score versus PCS score.
Table 2 SF-36 subscale and component score outcomes during acute TMS treatment (N = 307)

Notes: All data were computed using an observed case analysis.
P-value reflects within-group comparison of change from baseline to end of treatment performed using Student's T-Test.
MCS = Mental Component Summary score, PCS = Physical Component Summary score.
As reported elsewhere, 37.1% (N = 114) of the evaluable population achieved remission as defined by a clinician-rated end of acute treatment CGI-S score of ≤ 2.Reference Carpenter, Janicak and Aaronson21 This patient group showed a significantly greater improvement in MCS scores at the end of acute treatment [ie, a mean increase of 27.4 points (95% CI: 24.9–29.9)], compared with those patients who did not reach remission during acute TMS treatment [ie, a mean increase of 9.6 points (95% CI: 7.9–11.4)]. This same pattern of mental health improvement was evident when patient-reported outcomes (ie, PHQ-9 and IDS-SR) were used to define remission outcome. Patients who achieved remission on either the IDS-SR (defined as a total score < 15) or the PHQ-9 (defined as a total score < 5) showed a significantly greater improvement in MCS scores at the end of acute treatment compared with those patients who did not reach remission during acute TMS treatment [ie, for the IDS-SR: remitters showed a mean increase of 30.9 points (95% CI: 28.0–33.9) vs non-remitters who showed a mean increase of 11.1 points (95% CI: 9.4–12.7); the PHQ-9: remitters showed a mean increase of 30.1 points (95% CI: 27.3–32.8) vs non-remitters who showed a mean increase of 9.8 points (95% CI: 8.0–11.6)].
Finally, improvement in the SF-36 MCS and PCS scores in the study population was consistent across the range of depression symptom severity when grouped by baseline PHQ-9 total scores (Figures 1A–1C).
EQ-5D—Health Profile Summary scores, VAS General Health Perception, and Health Index scores
EQ-5D Health Profile Summary domain scores, VAS General Health Perception, and Health Index scores are summarized in Table 3. The largest treatment effects were observed on the domain scores associated with improvements in mental health (ie, anxiety/depression). Notably, statistically significant improvements in health function were seen in all domains, with the second largest treatment effects observed in the ability to carry out usual daily activities. Corresponding and statistically significant improvements were also seen in the General Health Perception and Health Index scores (Table 3).
Table 3 Summary of baseline and end of acute treatment EQ-5D scores: Health Profile Summary, VAS General Health Perception score, and Health Index Score (N = 307)

Notes: All data were computed using an observed case analysis.
P-value1 reflects the comparison of the proportion of patients reporting No Problems (Level 1) with the proportion of patients reporting Some or Extreme Problems (Levels 2 and 3 combined) from the baseline to end of treatment observation time points using a chi-square test.
P-value2 reflects within group comparison of change from baseline to end of treatment performed using Student's T-Test.
Using the same categorical end of treatment grouping of remission based on CGI-S total score noted above (CGI-S ≤ 2), the remitted patient group showed a significantly greater improvement in the Health Index score [ie, 0.26 points (95% CI: 0.22–0.29)] compared to the non-remitter group [ie, 0.13 points (95% CI: 0.10–0.17)]. This same pattern of improvement was evident when patient-reported outcomes were used to define remission outcome. Patients who achieved remission on either the IDS-SR (defined as a total score < 15) or the PHQ-9 (defined as a total score < 5) showed a significantly greater improvement in Health Index scores at the end of acute treatment compared with those patients who did not reach remission during acute TMS treatment [ie, the IDS-SR: remitters showed a mean increase of 0.28 points (95% CI: 0.24–0.32), vs non-remitters who showed a mean increase of 0.14 points (95% CI: 0.11–0.17); the PHQ-9: remitters showed a mean increase of 0.27 points (95% CI: 0.23–0.31), vs. non-remitters who showed a mean increase of 0.13 points (95% CI: 0.10–0.16)].
Finally, improvement in the Health Index score was consistent across the range of depression symptom severity in the population when grouped by baseline PHQ-9 total scores (Figures 1A–1C).
Influence of pre-treatment candidate predictors on QOL outcome measures
In the overall study population, none of the pre-treatment predictors of outcome met the defined criterion standard showing a significant main effect on outcome (data not shown). It is worth noting that ATR status had no effect on treatment outcome measures of functional status or QOL.
Discussion
Despite a variety of treatment options available for depression, recent studies find that pharmacoresistant patients continue to experience symptomatic and functional disability.Reference Trivedi, Rush and Wisniewski31, Reference Dunner, Rush and Russell46 This study explored the effect of an acute trial of TMS on health-related QOL outcomes in patients with treatment-resistant depression seen in clinical practice settings. To assess improvement in functional disability, we used the SF-36 and EQ-5D measures of QOL. These measures capture improvement in the health of depressed patients and differentiate between severity subgroups.Reference Mann, Gilbody and Richards37, Reference Gunther, Roick and Angermeyer47, Reference Sobocki, Ekman and Agren48 Our findings indicate that the amelioration of depressive symptoms is accompanied by an improvement in QOL. Both SF-36 and EQ-5D measures showed that remitted patients experienced a superior improvement in QOL compared with non-remitters, and patients with moderately severe to severe depression had the most robust improvement in QOL. In addition, our results suggest that TMS treatment has a relatively rapid beneficial impact on the QOL in these patients.
We observed that the mean MCS (16.6 ± 9.47) baseline score was more than 3 SDs below general population norms. This was slightly below the SF-36 MCS reported in other studies (ie, 2–3 SDs below norms).Reference Ware and Kosinski45, Reference Simon, Revicki and Grothaus49, Reference Garcia-Cebrian, Bauer and Montejo50 In contrast, the mean PCS baseline score (48 ± 11.91) was similar to the general population.
Similar to earlier antidepressant medication studies, improvements were much greater for mental health than for physical health dimensions.Reference Ware and Kosinski45, Reference Simon, Revicki and Grothaus49, Reference Walker, Streiner and Novosel51, Reference Reed, Monz and Perahia52 The vitality, social functioning, role-emotional, and mental health perceptions subscales all showed a statistically significant improvement of 1.2–1.5 SDs. The mean MCS score improved by 16.8 points to 33.5 (± 15.06), which is comparable to previous studies showing an improvement in the MCS scores following 9 months of treatment with paroxetine (15.8), fluoxetine (15.1), or sertraline (17.4).Reference Kroenke, West and Swindle53 The subpopulation of remitters showed a much more robust improvement of 27.4 to 30.9 points, reaching scores that are close to the general population norms and are only 0.13 to 0.55 SDs lower. In contrast, non-remitters showed only modest improvements of 9.6 to 11.1 points. While the Factors Influencing Depression Endpoints Research (FINDER) Study (a European, prospective, observational trial in 3,468 patients) reported that severely depressed patients had significantly worse SF-36 MCS outcomes, we observed that acute TMS treatment produced a pronounced QOL improvement in moderately severely and severely-ill patients compared with the mildly and moderately-ill subgroups.Reference Reed, Monz and Perahia52 At the end of acute treatment, the physical functioning, role-physical, bodily pain, and general health perceptions subscales all showed statistically significant but modest improvement (range from 0.38–0.72 SDs relative to the population norms), whereas the PCS score only minimally improved (0.19 SDs).
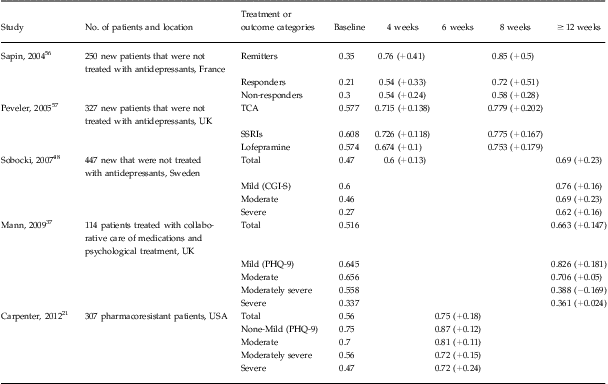
Of the five domains in the EQ-5D instrument, depression mainly impacted the anxiety/depression, usual activities, and pain/discomfort domains.Reference Sobocki, Ekman and Agren48 Our data found the same pattern in that 98.4% of our patients indicated problems in the anxiety/depression domain at baseline, followed by 85.7% and 60.6% of the patients who indicated problems in the usual activities and pain/discomfort domains, respectively. Conversion of the EQ-5D descriptive system to an index score translated into a baseline score of 0.56 (95% CI: 0.53–0.58). Comparable values are reported in other studies described in Table 4. At the end of acute treatment, we observed significant health gains. When we examined the index value scores of those patients in remission, there were larger health gains from baseline to end of treatment (range: 0.28–0.34, P < 0.0001) compared to those experienced by patients who did not achieve remission (range: 0.11–0.12, P < 0.0001).
Table 4 Summary of studies examining the change in EQ-5D index score following antidepressant treatment

Recent studies also demonstrate a correlation between depression and QOL.Reference Trivedi, Rush and Wisniewski31–Reference McCall, Rosenquist and Kimball33, Reference Mann, Gilbody and Richards37, Reference Yatham, Lecrubier and Fieve54, Reference ten Doesschate, Koeter and Bockting55 As mentioned previously, however, improvement in depressive symptoms may not accurately represent the overall treatment effect, and there are occasions when improvement in QOL lags behind symptomatic improvement.Reference Trivedi, Rush and Wisniewski31 To assess whether acute TMS treatment is correlated with disease burden, we performed correlation analyses and found a strong association between improvement in depressive symptoms and QOL as assessed by both the SF-36 and EQ-5D measures (data not shown). This is an important observation, indicating that TMS treatment can induce an improvement in depression and well-being in a relatively short time-frame (ie, 6 weeks). By comparison, most studies of antidepressant medication were typically 8 weeks or longer in duration.Reference Trivedi, Rush and Wisniewski9
Conclusion
In summary, treatment-resistant depressed patients experienced improvement in functional status and QOL following acute TMS treatment, which appears comparable to those produced by antidepressant medications. Most notably, severely depressed patients experienced the most robust improvement in a relatively short time-frame.
Discloures
Scott Aaronson has the following disclosures: Neuronetics, speaker's bureau, speakers fee; Genomind, consulting, consulting fee; Genomind, research support, research grant; Sunovion, speaker's bureau, speaker's fee. Dafna Bonneh-Barkay has the following disclosures: Neuronetics, employee, salary, stock options. Terrence Boyadjis has the following disclosures: Neuronetics, Inc., research support to the Institute of Pennsylvania (clinical trial), contract. David Brock has the following disclosures: Neuronetics, Inc. employee, salary, stock options. Ian Cook has the following disclosures: Covidien, consultant, consulting fees, research support, grant; Shire, research support, grant; Neuronetics, speaker's bureau, honoraria, research support, grant; Allergan, advisory board, consulting fee; Pfizer, advisory board, consulting fee; NeuroSigma, consultant, consulting fee, license of patents, stock options; NIH, research grant, grant, review committee, honoraria; VA, service on DSMB, honoraria. Linda Carpenter has the following disclosures: Neuronetics, research support to Butler, contract (clinical trial); Neosych, research support to Butler, contract (clinical trial); Corcept, consultant, honoraria; Takada/Lundbeck, consultant, consulting fee; Abbott, consultant, consulting fee; Johnson & Johnson, consultant, honoraria; Metronic, research support to Butler, contract (clinical trial). David Dunner has the following disclosures: Cyberonics, grant support, research support; Cervel, consultant, consultant fees; Neuronetics, grant support, research support, consultant, consultant fees; BristolMyersSquibb, consultant, consultant fees; Jazz, consultant, consultant fees. Mark Demitrack has the following disclosures: Neuronetics, Inc., employee, salary, stock options. Hugh Solvason has the following disclosures: Neuronetics, research support, research grant; St. Jude Medical, research support, research grant; Astrazeneca, research support, research grant; Roche, research support, research grant; Brain Resource, research support, research grant. Philip Janicak has the following disclosures: Cervel Neurotech, Inc., research support, grant; Janssen/OrthoMcNeil, research support, grant; Neuronetics, research support, grant, advisor, consultant; Novartis, research support, grant; Otsuka, research support, grant; The Research Foundation for Mental Hygiene, Inc., reserach support, grant; Karl Lanocha has the following disclosures: Neuronetics, research support, speaker's bureau.