Introduction
Pimavanserin is a highly selective serotonin 5-HT2A receptor inverse agonist/antagonist that was approved in the United States (US) in April 2016 for the treatment of hallucinations and delusions associated with Parkinson’s disease psychosis. 1 Because pimavanserin has no measurable activity at dopaminergic, histaminergic, adrenergic, or muscarinic receptors, this would predict a favorable tolerability profile in that motor symptoms would not be expected to worsen, and urinary retention, constipation, sedation, weight gain, akathisia, and postural hypotension would not be expected obstacles to using pimavanserin. The recommended dose is 34 mg taken once daily, any time of the day, with or without food. Titration is not necessary.
Parkinson’s disease (PD) itself is a chronic, progressive, neurodegenerative disease characterized by both motor and non-motor features,Reference DeMaagd and Philip 2 – Reference DeMaagd and Philip 6 with a prevalence that increases with age.Reference Pringsheim, Jette, Frolkis and Steeves 7 The Parkinson’s Disease Foundation reports that PD affects about a million people in the US, with 60,000 diagnosed each year. 8 Parkinson’s disease psychosis (PDP) is a common non-motor neuropsychiatric manifestation of PD. Up to 60% of patients with PD experience psychotic symptoms for at least a month at some point during the course of their illness.Reference Fénelon, Soulas, Zenasni and Cleret de Langavant 9
PDP is characterized by hallucinations (often visual) and delusions (typically paranoid, such as spousal infidelity), as well as illusions and a false sense of presence.Reference Ravina, Marder and Fernandez 10 The burden of PDP is substantial for both patient and caregiver,Reference Schrag, Hovris, Morley, Quinn and Jahanshahi 11 with symptoms of psychosis being a common reason for hospital admission,Reference Klein, Prokhorov, Miniovitz, Dobronevsky and Rabey 12 nursing home placement,Reference Aarsland, Larsen, Tandberg and Laake 13 and increased mortality.Reference Goetz and Stebbins 14 Moreover, comorbidities with depressive disorder, sleep–wakefulness problems, and cognitive impairment/dementia are common.Reference Lee and Weintraub 15
Although several risk factors have been identified as being associated with PDP, including the use of dopaminergic medications used to treat PD,Reference Ravina, Marder and Fernandez 10 , Reference Zahodne and Fernandez 16 PDP can develop in the absence of dopamine replacement therapy.Reference Ravina, Marder and Fernandez 10 This latter observation can be explained by disruption in serotonin signaling caused by the accumulation of Lewy bodies in the cerebral cortex, with resultant upregulation of cortical serotonin 5-HT2A receptors in the temporal cortex and in visual pathways, hypothetically causing visual hallucinations,Reference Stahl 17 , Reference Ballanger, Strafella and van Eimeren 18 akin to what can be observed with exposure to potent 5-HT2 agonists such as LSD,Reference Sadzot, Baraban and Glennon 19 and it is noteworthy that pimavanserin acts as an inverse agonist/antagonist at these receptors, potentially explaining its therapeutic effects in PDP. 1 At the present time, pimavanserin is the only agent approved by the US Food and Drug Administration (FDA) for the treatment of hallucinations and delusions associated with PDP.
Our study aim was to review the evidence base for pimavanserin in the treatment of PDP using the metrics of evidence-based medicine—namely, number needed to treat (NNT), number needed to harm (NNH), and likelihood to be helped or harmed (LHH)—in order to better place this intervention into clinical perspective.Reference Citrome 20 – Reference Citrome and Kantrowitz 24
Methods
Calculated are NNT and NNH and their respective 95% confidence intervals (CIs) for the outcomes of interest listed below. NNT and NNH are measures of effect size and indicate how many patients would need to be treated with one agent instead of the comparator in order to encounter one additional outcome of interest. Lower NNTs are evidenced when there are large differences between the interventions in question. For example, an NNT of 2 would be a very large effect size, as a difference is encountered after treating just 2 patients with one of the interventions versus the other. An NNT of 50 would mean little difference between the two interventions, as it would take treating 50 patients to encounter a difference in outcome. NNH is used when referring to undesirable events. A useful medication is one with a low NNT and a high NNH when comparing it with another intervention; a low NNT and a high NNH would mean one is more likely to encounter a benefit than harm. A rule of thumb is that single-digit NNTs for efficacy measures suggest that the intervention has potentially useful advantages, and that double-digit or higher NNHs for adverse outcomes indicate that the intervention is potentially tolerable. NNT and NNH can be contrasted using the ratio of LHH. An LHH greater than 1 would mean that the likelihood to be helped is greater than the likelihood to be harmed. For an LHH less than 1, the reverse is true.
Data sources
Data were extracted from the clinical trial databases of the double-blind placebo-controlled studies of pimavanserin in persons with PDP. 25 – 33 The studies are summarized in Supplemental Tables 1 and 2. These studies were 6 weeks in duration and fixed-dose, with the exception of study ACP-103-006, which was 4 weeks in duration and flexible-dose. The principal clinical trial of interest, study ACP-103-020, was the pivotal study of pimavanserin that led to product approval.Reference Cummings, Isaacson and Mills 28 In that Phase III 6-week trial, 199 subjects were randomized 1:1 to receive pimavanserin 34 mg daily (equivalent to pimavanserin tartrate 40 mg as published in the original study report) or matching placebo. Subject disposition and efficacy outcomes for this study have been previously disclosed 25 or published.Reference Cummings, Isaacson and Mills 28 Additional analyses were conducted by pooling study results from other trials, as noted below.
Table 1 Efficacy outcomes for study ACP-103-020
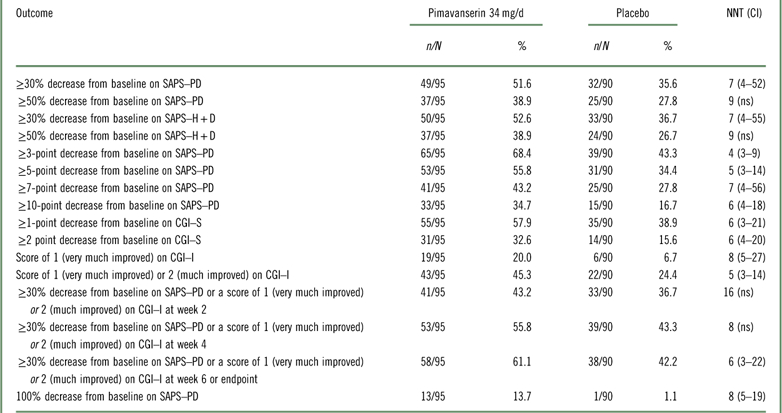
CGI–I=Clinical Global Impression Scale–Improvement; CGI–S=Clinical Global Impression Scale–Severity; n=numerator; N=denominator (randomized subjects who received at least one dose of study drug and had at least one postbaseline assessment); ns=not significant; SAPS–H+D=Scale for the Assessment of Positive Symptoms hallucinations and delusions items; SAPS–PD=Scale for the Assessment of Positive Symptoms adapted for Parkinson’s disease.
Table 2 Efficacy outcomes for studies ACP-103-020 and ACP-103-012 (pooled data for pimavanserin 34 mg/d and placebo [US sites for ACP-103-012])
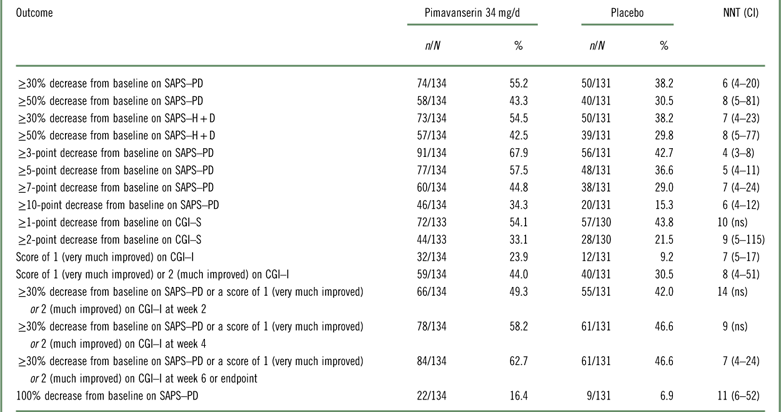
CGI–I=Clinical Global Impression Scale–Improvement; CGI–S=Clinical Global Impression Scale–Severity; n=numerator; N=denominator (randomized subjects who received at least one dose of study drug and had at least one postbaseline assessment); ns=not significant; SAPS–H+D=Scale for the Assessment of Positive Symptoms hallucinations and delusions items; SAPS–PD=Scale for the Assessment of Positive Symptoms adapted for Parkinson’s disease.
Efficacy outcomes
Measured were response and remission (defined below) at 6 weeks (last observation carried forward [LOCF]) for subjects receiving pimavanserin 34 mg/d or placebo. The study population for these analyses was the number of randomized subjects who received at least one dose of study drug and had a baseline and at least one postbaseline assessment on the efficacy outcome of interest. The reporting of categorical outcomes differs from that described in the product labeling in that the latter used the convention that subjects with missing values were counted as nonresponders. 1 In addition to pivotal study ACP-103-020, efficacy outcomes from study ACP-103-012 for the 34 mg/d dose were also examined using data collected from study sites in the US, outside the US (Europe and India), and combined, and pooled with the results observed in study ACP-103-020.
Response was defined using several different thresholds of symptom improvement: ≥30% and ≥50% decreases from baseline on the Scale for the Assessment of Positive Symptoms adapted for Parkinson’s Disease (SAPS–PD)Reference Voss, Bahr, Cummings, Mills, Ravina and Williams 34 and the Scale for the Assessment of Positive Symptoms hallucinations and delusions items (SAPS–H+D);Reference Voss, Bahr, Cummings, Mills, Ravina and Williams 34 ≥3-, 5-, 7-, and 10-point decreases from baseline on the SAPS–PD; ≥1- and 2-point decreases from baseline on the Clinical Global Impression–Severity Scale (CGI–S);Reference Guy 35 a score of 1 (very much improved compared to baseline) on the Clinical Global Impression–Improvement Scale (CGI–I);Reference Guy 35 a score of 1 (very much improved) or 2 (much improved) on the CGI–I; a ≥30% decrease from baseline on the SAPS–PD or a score of 1 (very much improved) or 2 (much improved) on the CGI–I. For the latter threshold, response was also ascertained for the timepoints of weeks 2, 4, and 6.
Remission was defined by a reduction of 100% from baseline on the primary outcome measure.
The above measures of response and remission were assessed separately for all subjects in study ACP-103-020 whose baseline ratings on the SAPS–PD were greater than or equal to the median SAPS–PD score, and with data pooled from subjects who received pimavanserin 34 mg/d or placebo in study ACP-103-012, where baseline ratings on the SAPS–PD were greater than or equal to the median SAPS–PD score in that study.
Tolerability outcomes
Several tolerability and safety outcomes occurring at any time in the study were assessed. The study population for these analyses was the number of randomized subjects who had received at least one dose of study drug and who had a baseline and at least one postbaseline assessment on the tolerability or safety measure of interest (not applicable for spontaneously reported adverse events where the study population was the number of all randomized subjects who received at least one dose of study drug). Outcomes for study ACP-103-020 were examined first, and the data were then pooled using data from studies ACP-103-012 (pimavanserin 8.5 and 34 mg), ACP-103-014 (8.5 and 17 mg), and ACP-103-006 (flexible dose 17–51 mg/d). The reporting of adverse reactions differs from that described in the product labeling in that the latter contrasted only the patients receiving pimavanserin 34 mg/d or placebo in the three 6-week studies, thus excluding other doses of pimavanserin and also excluding those patients receiving placebo in the 4-week study (ACP-103-006).
Measured were spontaneously reported adverse events (threshold of >2% in any pimavanserin treatment group); discontinuation because of an adverse event; worsening from baseline on the Unified Parkinson Disease Rating Scale (UPDRS) Parts II and IIIReference Fahn and Elton 36 at early termination/endpoint (defined as worse than baseline, ≥5% worse from baseline, ≥10% worse from baseline, ≥20% worse from baseline); increase in weight from baseline by ≥7% at early termination/endpoint and decrease in weight from baseline of ≥7% at early termination/endpoint; orthostatic hypotension (defined as the decrease in systolic [≥20 mmHg] or diastolic [≥15 mmHg] blood pressure or an increase in pulse rate of ≥20 bpm from 5 minutes supine to 1 minute standing at the same visit) based on vital sign measurement; electrocardiogram (ECG) QTcF interval >450 ms, >500 ms, or a change in QTcF ≥60 ms over baseline.
Formulae used
▪Attributable Risk Increase (ARI)=(incidence on medication)− (incidence on placebo)=f 1−f 2
▪ The CI was calculated by:
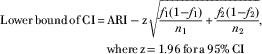 $$\eqalign { {\rm Lower}\,{\rm bound}\,{\rm of}\,{\rm CI}\,{\equals}\, & {\rm ARI}-{\rm z}\sqrt {{{f_{1} (1{\minus}f_{1} )} \over {n_{1} }} {\plus}{{f_{2} (1{\minus}f_{2} )} \over {n_{2} }}}, \cr& {\rm where}\,{\rm z\,{\equals}\,1}{\rm .96}\,{\rm for}\,{\rm a\,95%}\,{\rm CI}$$
$$\eqalign { {\rm Lower}\,{\rm bound}\,{\rm of}\,{\rm CI}\,{\equals}\, & {\rm ARI}-{\rm z}\sqrt {{{f_{1} (1{\minus}f_{1} )} \over {n_{1} }} {\plus}{{f_{2} (1{\minus}f_{2} )} \over {n_{2} }}}, \cr& {\rm where}\,{\rm z\,{\equals}\,1}{\rm .96}\,{\rm for}\,{\rm a\,95%}\,{\rm CI}$$
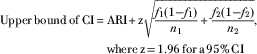 $$\eqalign { {\rm Upper}\,{\rm bound}\,{\rm of}\,{\rm CI}\,{\equals}\, & {\rm ARI}{\plus}{\rm z}\sqrt {{{f_{1} (1{\minus}f_{1} )} \over {n_{1} }} {\plus}{{f_{2} (1{\minus}f_{2} )} \over {n_{2} }}},\; \cr& {\rm where}\,{\rm z\,{\equals}\,1}{\rm .96}\,{\rm for}\,{\rm a\,95%}\,{\rm CI}$$
$$\eqalign { {\rm Upper}\,{\rm bound}\,{\rm of}\,{\rm CI}\,{\equals}\, & {\rm ARI}{\plus}{\rm z}\sqrt {{{f_{1} (1{\minus}f_{1} )} \over {n_{1} }} {\plus}{{f_{2} (1{\minus}f_{2} )} \over {n_{2} }}},\; \cr& {\rm where}\,{\rm z\,{\equals}\,1}{\rm .96}\,{\rm for}\,{\rm a\,95%}\,{\rm CI}$$
▪ NNT (or NNH) = 1/ARI, and rounded up to the next highest whole number
▪ The CI for the NNT (or NNH) was calculated by taking the reciprocal of the lower and upper bounds of the CI for the ARI, and rounded up to the next highest whole number
▪ LHH=NNH/NNT
Results
Efficacy outcomes
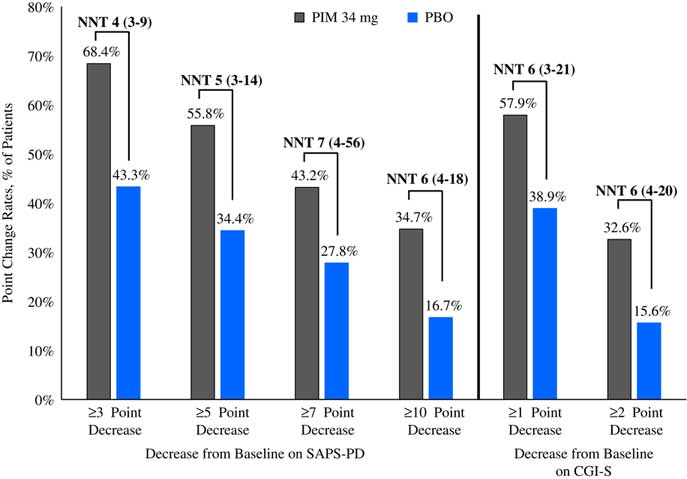
Please refer to Table 1 and Figures 1–3. In the 6-week pivotal trial of pimavanserin 34 mg/d versus placebo (ACP-103-020), the response observed with pimavanserin as defined by a ≥30% decrease from baseline on the SAPS–PD, a ≥30% decrease from baseline on the SAPS–H+D, a ≥5-point decrease from baseline on the SAPS–PD, a ≥1-point decrease from baseline on the CGI–S, and a ≥30% decrease from baseline on the SAPS–PD or a score of 1 (very much improved) or 2 (much improved) on the CGI–I, yielded similar rates and ranged from 51.6 to 61.1%. Contrasting these rates with those observed with placebo (34.4 to 42.2%), NNT values ranged from 5 to 7. A more liberal definition of response, operationalized as a ≥3-point decrease from baseline on the SAPS–PD, yielded response rates of 68.4% for pimavanserin-treated patients versus 43.3% for placebo-treated patients, resulting in an NNT of 4, with a narrow CI of 3–9. More conservative definitions of response, including a ≥50% decrease from baseline on the SAPS–PD, a ≥50% decrease from baseline on the SAPS–H+D, a ≥7- or ≥10-point decrease from baseline on the SAPS–PD, a ≥2-point decrease from baseline on the CGI–S, and a score of 1 (very much improved) or 2 (much improved) on the CGI–I, revealed lower rates, ranging from 32.6 to 45.3% for pimavanserin compared to 15.6 to 27.8% for placebo, resulting in NNT values ranging between 5 to 9. A more robust response (or remission) as defined by either a score of 1 (very much improved) on the CGI–I or a 100% decrease from baseline on the SAPS–PD was less frequently observed, with rates ranging from 13.7 to 20.0% for pimavanserin-treated patients versus 1.1 to 6.7% for placebo-treated patients, yielding an NNT value (using either definition of robust response or remission) of 8.
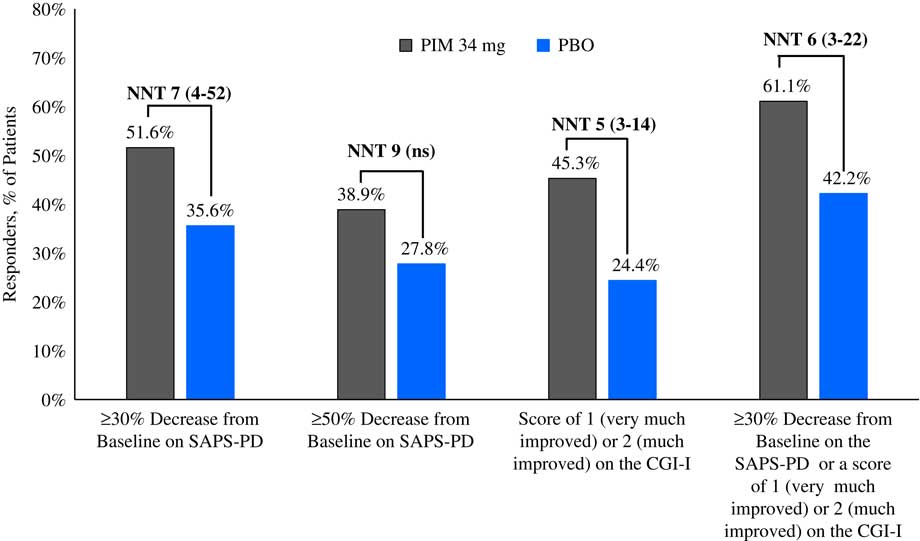
Figure 1 Response rates for pimavanserin 34 mg/d (PIM) vs. placebo (PBO), study ACP-103-020.
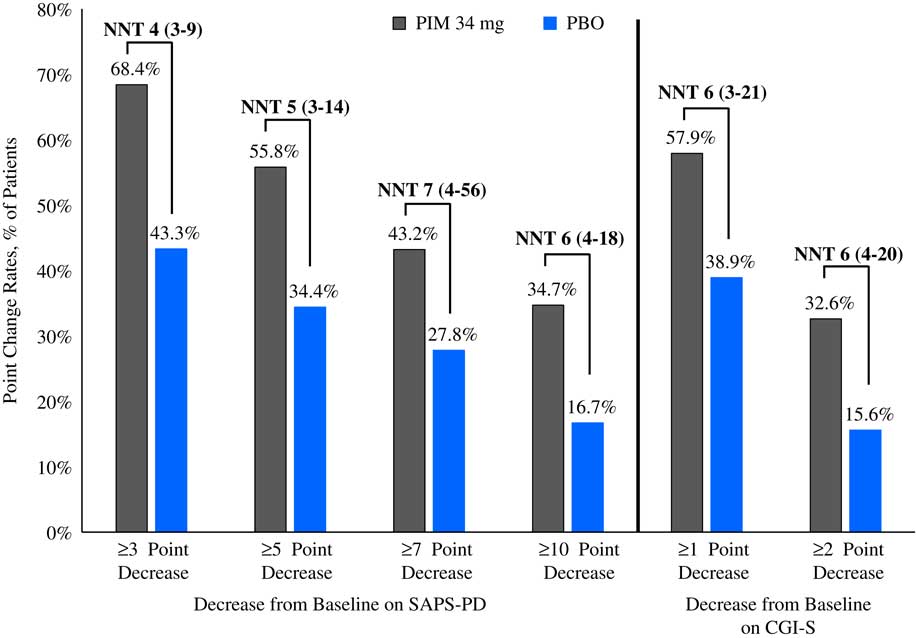
Figure 2 Response rates as measured by point change for pimavanserin 34 mg/d (PIM) vs. placebo (PBO), study ACP-103-020.
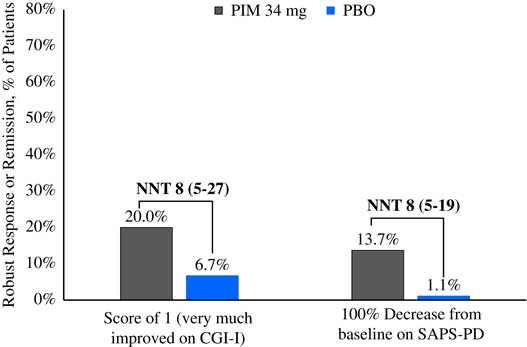
Figure 3 Robust response/remission rates for pimavanserin 34 mg/d (PIM) vs. placebo (PBO), study ACP-103-020.
Response over time, as assessed using the definition of a ≥30% decrease from baseline on the SAPS–PD or a score of 1 (very much improved) or 2 (much improved) on the CGI–I at weeks 2, 4, and 6 demonstrated that the NNT became more robust (i.e., lower in value) over time; however, statistical significance was observed only at week 6/Endpoint.
The results examining pooled data from study ACP-103-020 and the pimavanserin 34 mg/d and placebo arms at the US study sites from ACP-103-012 were similar to the above (Table 2 and Supplemental Figures 1–3). When all study sites from ACP-103-012 were included (i.e., when adding the non-US study sites in ACP-103-012 where the primary outcome measure was assessed by a blinded site-based rater instead of being assessed by a blinded centralized rater via a live video interview, as was done at US sites and at all sites in pivotal study ACP-103-020), the effect sizes decreased (i.e., NNT values were higher) (Supplemental Table 3).
Examining more severely ill subjects in study ACP-103-020, as defined by a baseline SAPS–PD score ≥ the median score of 14, the NNT values for response were either essentially unchanged or somewhat less robust (Supplemental Table 4). Pooling these data with data from all sites in study ACP-103-012 and where the median SAPS–PD score was ≥ the median score of 11.5, effect sizes generally decreased (Supplemental Table 5).
Tolerability outcomes
In the 6-week pivotal trial of pimavanserin 34 mg/d versus placebo (ACP-103-020), overall tolerability, as defined by rates of discontinuation because of an adverse event, evidenced an NNH of 16 in favor of placebo; however, this was not statistically significant (Table 3, Supplemental Figure 4). Because of the relatively small sample size, events that have a relatively low incidence will not demonstrate statistically significant differences when comparing pimavanserin with placebo. The only tolerability outcomes that did demonstrate a statistically significant difference were the adverse event of hallucination (rates of 6.7 and 1.1% for pimavanserin and placebo, respectively), where the NNH was 18, and orthostatic hypotension at any postbaseline timepoint demonstrating a lower incidence for pimavanserin (33.0 vs. 48.4%), yielding an NNH value of –7 (which can be interpreted as an NNT advantage of 7 in favor of pimavanserin). In summary, NNH values regarding tolerability outcomes were consistently ≥10, and for the most part not statistically significant, or at times showing an advantage for pimavanserin.
Table 3 Safety and tolerability outcomes for study ACP-103-020
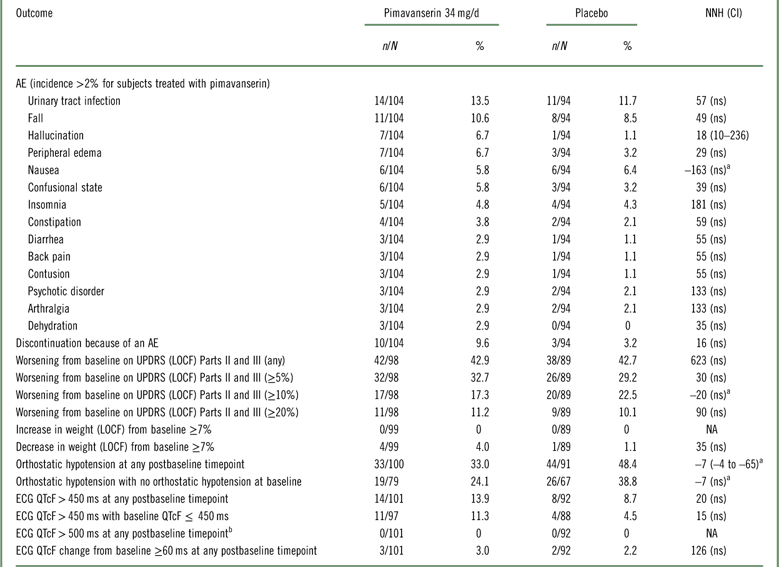
a A “negative” NNH results from when the rate of the safety or tolerability outcome is higher for placebo than for pimavanserin. In these instances, with the exception of orthostatic hypotension at any postbaseline timepoint, the results were not statistically significant.
b No subjects had a baseline ECG QTcF>500 ms.
AE=adverse event; ECG=electrocardiogram; LOCF=last observation carried forward; n=numerator; N=denominator (for safety outcomes: all randomized subjects who received at least one dose of study drug and who have at least one postbaseline assessment on the tolerability or safety measure of interest; not applicable for spontaneously reported adverse events where the study population is the number of all randomized subjects who have received at least one dose of study drug ); NA=not applicable; ns=not significant; UPDRS=Unified Parkinson’s Disease Rating Scale.
Tolerability outcomes were examined by pooling data from study ACP-103-020 and the pimavanserin 34 mg/d and placebo arms from ACP-103-012 (Table 4, Supplemental Figure 4). Overall tolerability, as defined by rates of discontinuation because of an adverse event, evidenced an NNH of 21. Other NNH values that were statistically significant were observed for the outcomes of peripheral edema (NNH 21) and ECG QTcF>450 ms for subjects with a baseline QTcF ≤ 450 ms (NNH 18). No subject had a postbaseline QTcF>500 ms in these two studies.
Table 4 Safety and tolerability outcomes for studies ACP-103-020 and ACP-103-012 (pooled data for pimavanserin 34 mg/d and placebo)
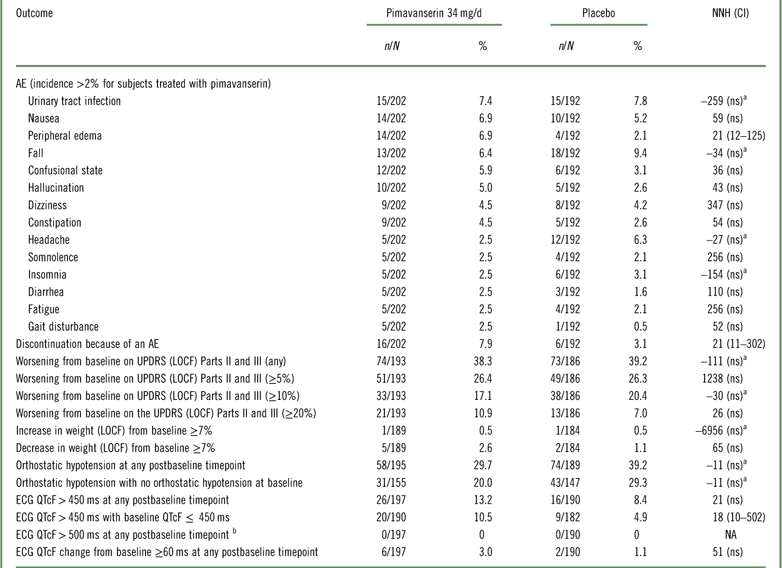
a A “negative” NNH results from when the rate of the safety or tolerability outcome is higher for placebo than for pimavanserin. In these instances the results were not statistically significant.
b No subjects had a baseline ECG QTcF>500 ms.
AE=adverse event; ECG=electrocardiogram; LOCF=last observation carried forward; n=numerator; N=denominator (for safety outcomes: all randomized subjects who received at least one dose of study drug and who have at least one postbaseline assessment on the tolerability or safety measure of interest; not applicable for spontaneously reported adverse events where the study population is the number of all randomized subjects who have received at least one dose of study drug); NA=not applicable; ns=not significant; UPDRS=Unified Parkinson’s Disease Rating Scale.
Pooling all tolerability outcome data for all doses of pimavanserin from all four double-blind randomized clinical trials demonstrated comparable results, as described above (Supplemental Table 6, Supplemental Figure 4). Overall tolerability, as defined by rates of discontinuation because of an adverse event, evidenced an NNH of 33 in favor of placebo; however, this was not statistically significant. Two tolerability outcomes were statistically significant in favor of placebo: QTcF>450 ms for subjects with a baseline QTcF ≤ 450 ms (NNH 25; 1 subject receiving pimavanserin and 1 subject receiving placebo had a QTcF>500 ms at any postbaseline timepoint), and decrease in weight by ≥7% from baseline (NNH 43). Favoring pimavanserin were the outcomes of orthostatic hypotension at any postbaseline timepoint and orthostatic hypotension with no orthostatic hypotension at baseline, with both having an NNH value versus placebo of –12 (i.e., an NNT of 12 in favor of pimavanserin). Thus, when pooling all available data, NNH values regarding tolerability outcomes were consistently ≥10, and for the most part not statistically significant, or at times showing an advantage for pimavanserin.
The tolerability pattern of pimavanserin is different from that of the second-generation antipsychotics. Of note, NNH values for pimavanserin (all doses) versus placebo for somnolence was 138 and for weight gain ≥7% from baseline, –594 (Supplemental Table 6), denoting that these events were not commonly associated with this agent. Akathisia was not observed. In addition, there were no observed deleterious effects on mood.
Likelihood to be helped or harmed
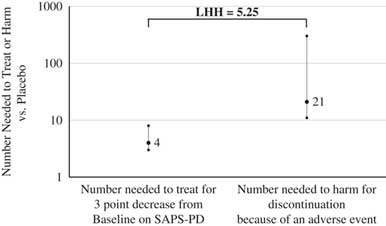
The metric of LHH answers the question of how often one would encounter a benefit versus harm. In general, the NNT values for response for pimavanserin 34 mg/d versus placebo were <10, and all of the tolerability outcomes, except for orthostatic hypotension (for which pimavanserin may have a protective effect), evidenced NNH values ≥10, yielding LHH values >1. By any measure, the chances of encountering a benefit are more likely than encountering a harm. A useful definition of response is a ≥3-point decrease from baseline on the SAPS–PD. Prior work by Voss and colleaguesReference Voss, Bahr, Cummings, Mills, Ravina and Williams 34 demonstrated that a 2.33-point change on the SAPS–PD corresponds to a clinically meaningful 1-unit change on the CGI–I. The NNT is 4 for a ≥3-point decrease from baseline on the SAPS–PD for pimavanserin 34 mg/d versus placebo when examining the results of the pivotal ACP-103-020 study, or when pooling those data with that from the US study sites for study ACP-103-012 (i.e., where the primary efficacy outcome was measured using a methodology similar to that of study ACP-103-020). The NNH is 21 for the overall tolerability metric of discontinuation because of an adverse event from all the pooled data for pimavanserin 34 mg/d versus placebo from studies ACP-103-020 and ACP-103-012. The resulting LHH is 21/4=5.25, which can be interpreted as pimavanserin 34 mg/d, is about five times more likely to result in a response (≥3-point decrease from baseline on the SAPS–PD) than discontinuation because of an adverse event, with no overlap noted in the respective CIs for the NNT and NNH values used for this specific calculation (Figure 4).
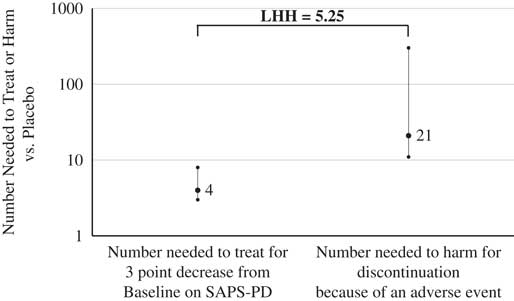
Figure 4 Number needed to treat for response and number needed to harm for discontinuation because of an adverse event, and (95% confidence intervals, for pimavanserin 34 mg/d vs. placebo, and likelihood to be helped or harmed.
Discussion
In general, efficacy outcomes for response using several different definitions yielded NNT values for pimavanserin 34 mg/d versus placebo of <10, denoting that pimavanserin is a potentially efficacious intervention.Reference Citrome and Ketter 23 NNH values regarding tolerability outcomes were consistently ≥10, denoting that pimavanserin is a potentially tolerable intervention;Reference Citrome and Ketter 23 in addition, for the most part, the NNH estimates were not statistically significant and/or demonstrated an advantage for pimavanserin. Many successful psychotropic medications for several indications have NNT values between 3 and 9 for clinically relevant definitions of response.Reference Pinson and Gray 22 The lower the NNT, the more often desired outcomes are encountered. In contrast, higher NNH values are optimal, so that adverse outcomes are seldom encountered.Reference Citrome and Ketter 23
These results were consistent with NNT and NNH estimates calculated from data available in product labeling and from the sponsor’s FDA Psychopharmacologic Drugs Advisory Committee briefing document. 1 , 25 From those data, response at week 6, as defined by an SAPS–PD point reduction ≥3 and where subjects with missing values counted as nonresponders, was observed in 62/95 (65%) of subjects receiving pimavanserin versus 38/90 (42%) for placebo, for an NNT versus placebo of 5 (CI=3–12). Regarding adverse reaction rates for pimavanserin 34 mg/d versus placebo from the 6-week studies, peripheral edema was observed in 14/202 (7%) for pimavanserin versus 5/231 (2%) for placebo, for an NNH of 21 (CI=12–127), and confusional state, seen in 12/202 (6%) for pimavanserin against 6/231 (3%) for placebo, for an NNH of 30 (not statistically significant). The discontinuation rate due to an adverse event was 16/202 (8%) for pimavanserin versus 10/231 (4%) for placebo, yielding an NNH of 28 (not statistically significant).
Effect sizes for the relevant clinical outcomes are also consistent with a metaanalysis reported by Yasue and colleagues,Reference Yasue, Matsunaga, Kishi, Fujita and Iwata 37 where pimavanserin significantly decreased SAPS–H+D scores compared to placebo; was associated with less orthostatic hypotension than placebo; and that there were no significant differences in rates of all-cause discontinuation, adverse events, and death, or UPDRS Parts II and III scores, nor in incidences of individual adverse events (other than orthostatic hypotension) between the pimavanserin and placebo groups.Reference Yasue, Matsunaga, Kishi, Fujita and Iwata 37
The clinical usefulness of pimavanserin can be contrasted with other interventions, notably second-generation antipsychotics (SGAs), where double-blind randomized controlled clinical trial data are available. To provide indirect comparisons with the results observed here for pimavanserin, double-blind placebo-controlled clinical trials were identified through the US National Library of Medicine’s PubMed.gov resourceReference Breier, Sutton and Feldman 38 – Reference Fernandez, Okun and Rodriguez 50 (Supplemental Tables 7–10). The clozapine studies demonstrated statistically significant and clinically relevant reductions in psychotic symptoms when compared with placebo, but this was not the case in the olanzapine or quetiapine studies. Olanzapine-treated patients also demonstrated worsening of motor symptoms. Although quetiapine is reasonably well-tolerated regarding motor symptoms, in two of the trials of quetiapine, placebo was associated with a numerical advantage over quetiapine on the efficacy measures of interest.Reference Ondo, Tintner, Voung, Lai and Ringholz 44 , Reference Rabey, Prokhorov, Miniovitz, Dobronevsky and Klein 47 In a meta-analysis by Frieling et al.Reference Frieling, Hillemacher, Ziegenbein, Neundörfer and Bleich 40 the authors concluded that only clozapine can be fully recommended for the treatment of PDP and that olanzapine should not be used for this indication. Although the effect size for the primary efficacy measure in the positive pivotal study for pimavanserin was 0.5,Reference Cummings, Isaacson and Mills 28 and thus not as robust as that observed with clozapine, pimavanserin does not require the hematological monitoring that is compulsory for clozapine, and the clinical trials of pimavanserin did not find evidence of an increased rate of sedation, orthostatic hypotension, or anticholinergic and metabolic adverse effects that are commonly observed with clozapine. Clozapine remains “off-label” in the US for PDP, and because of its tolerability and safety profile and its requirement for weekly blood testing (for the first 6 months, then biweekly for the next 6 months, then monthly thereafter), its actual use for PDP is uncommon.Reference Hack, Fayad and Monari 51 , Reference Weintraub, Chen, Ignacio, Mamikonyan and Kales 52
Limitations
The data analyzed in this study are limited to dichotomous outcomes. The results may not be generalizable to patients outside the confines of a clinical trial. Reasons for clinical trial discontinuation can be complex, so that the NNH for discontinuation due to adverse effects in the study may not always generalize to overall tolerability in clinical practice. The brief (4–6 week) durations of the available controlled studies of pimavanserin limit the sensitivity of calculating NNH for delayed adverse outcomes, and the relatively small sample sizes of the studies limit the sensitivity of calculating NNH for uncommon adverse outcomes and subpopulation effects.
Conclusions
NNT values for pimavanserin 34 mg/d versus placebo for several definitions of clinical response are generally <10, and for some outcomes the NNT is as robust as 4. NNH values for tolerability outcomes with pimavanserin 34 mg/d (or for doses that range from 8.5 to 51 mg/d) are >10, and/or are not statistically significant, and/or show an advantage for pimavanserin (such as for postural hypotension). In terms of LHH, pimavanserin 34 mg/d is about five times more likely to result in a clinical response as measured by a ≥3-point decrease from baseline on the SAPS–PD rather than discontinuation due to an adverse event. Using the metrics of NNT, NNH, and LHH, pimavanserin 34 mg/d for the treatment of PDP appears to have a compelling benefit/risk profile.
Disclosures
Leslie Citrome reports financial support from ACADIA Pharmaceuticals Inc. during the conduct of the study; personal fees from ACADIA, Alexza, Alkermes, Allergan, AstraZeneca, Avanir, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Forum, Genentech, Intra-Cellular Therapeutics, Janssen, Jazz, Lundbeck, Medivation, Merck, Mylan, Neurocrine, Novartis, Noven, Otsuka, Pfizer, Reckitt Benckiser, Reviva, Shire, Sunovion, Takeda, Teva, Valeant, and Vanda outside the submitted work.
James Norton, Kathy Chi-Burris, and George Demos are employees of ACADIA Pharmaceuticals Inc.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1092852917000736