In a recently published article by Grassi et al,Reference Grassi, Cacchelli and Mazzocato 1 they characterized early-onset obsessive–compulsive disorder (OCD) biotype and phenotype. They highlighted the role of 5HT3 receptors in OCD and that blockade of these receptors boosts serotonergic tone by disinhibiting glutamergic terminals.Reference Stahl 2 This can be pharmacologically achieved by repurposing “setrons.” A prototype of this group is ondansetron.
Ondansetron is a first-line antiemetic. It blocks 5HT3 receptors. Ondansetron exerts its effects in gastrointestinal tract (GIT) and chemoreceptor trigger zone (CTZ). It is generally very well tolerated barring occasional constipation. QTc prolongation has been reported with IV ondansetron and the maximum IV dose is now 16 mg (instead of 32 mg previously). An oral disintegrating tablets (ODT) formulation is available on the market and given sublingual (SL) for faster onset of action. It is a substrate for CYP 3A4.
Interestingly, ondansetron has been used off-label to address a multitude of psychiatric indications (Table 1). It should be borne in mind that the level of evidence supporting the use of ondansetron in these off-label indications is highly variable, and, hence, clinical acumen is necessary for its proper use and placement in real-life psychiatric practice. Here, we briefly touch on some of these indications while examining the extant evidence.
Table 1. Ondansetron’s Therapeutic Potential
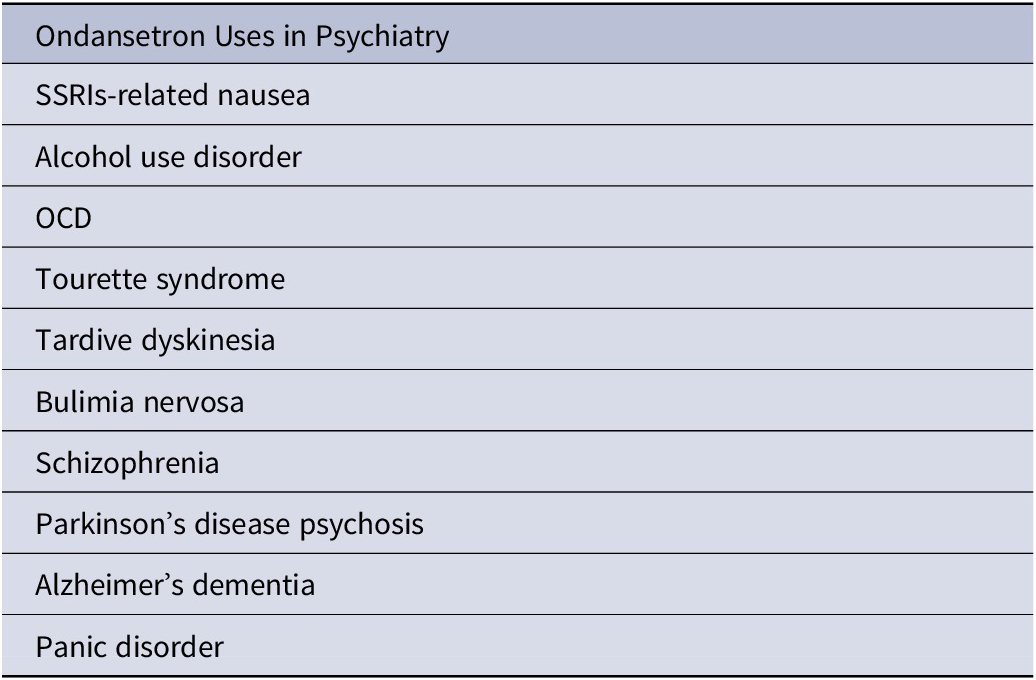
Abbreviations: OCD, obsessive–compulsive disorder; SSRI, selective serotonin reuptake inhibitor.
Selective serotonin reuptake inhibitors (SSRIs)-related nausea
Nausea associated with SSRIs and serotonin–norepinephrine reuptake inhibitors is believed to be due to excessive stimulation of 5-HT3 receptors. It emerges in about 25% of patients early on during treatment, and although it typically wanes after 2 weeks or so in most cases, it may persist in one-third of patients.Reference Montgomery 3 “Setrons” help SSRIs nausea by blocking the postsynaptic 5-HT3 receptors. Clinicians should be vigilant when adding ondansetron to antidepressants that are potent inhibitors to CYP-450 (eg, paroxetine) which can risk QTc prolongation. Similarly, by virtue of 5-HT3 blockade, low dose of mirtazapine can help easing out antidepressant nausea. In the same vein, olanzapine, with 5-HT3 blockade, has been effective to address commonly encountered duloxetine-associated nausea.
Of related interest, ondansetron has been reported to help with venlafaxine discontinuation symptoms (nausea, GIT upset, headache, etc.).
Alcohol use disorder
Ethanol administration may facilitate the release of serotonin within the nucleus accumbens. This and other mechanisms may be involved in the enhancement of thr function of 5-HT3A receptor produced by exposure to ethanol. Administration of 5-HT3A antagonists may inhibit ethanol-induced dopamine release in ventral tegmental area (VTA) and nor-adrenalin (NA). As such, ondansetron has been demonstrated to reduce drinking behavior among patients with early-onset alcoholism (prior to 25 years of age). Drinking was reduced in most of the individuals with the L/L genotype at 5-HTTLPR.Reference Pettinati, Oslin and Decker 4
Given that, 5-HT3A receptor may also be involved in the reinforcing effects of inhalants, it is plausible that ondansetron and other agents that antagonize this complex receptor may reduce or dampen the reward produced by inhalant use.
Obsessive–compulsive disorder
There have been at least six trials that have examined a short-term (8-12 weeks) role for ondansetron in patients with OCD. These include one placebo-controlled crossover trial (N = 11); one uncontrolled monotherapy trial (N = 8); two low-dose (0.5-1.0 mg/day), uncontrolled augmentation trials in patients who did not respond adequately to ongoing or earlier treatments (pooled N = 35); and two moderate- to high-dose (4-8 mg/day) randomized, placebo-controlled augmentation trials in patients with undocumented past treatment history (pooled N = 88). Ondansetron was modestly effective in the uncontrolled trials and strikingly effective in the controlled trials. Ondansetron was also very well tolerated in all of the studies. At best, ondansetron (1-8 mg/day) may be considered an experimental serotonin reuptake inhibitor (SRI) augmentation agent in OCD patients for whom augmentation with an atypical antipsychotic drug is problematic.
Tourette syndrome
Ondansetron (8-24 mg/day) showed efficacy for a self-report but not an observer-rated measure of clinical improvement in a 3-week double-blind randomized controlled trial (RCT) in patients aged 12 to 46 years with Tourette syndrome.
Tardive dyskinesia
Twenty patients with schizophrenia who had neuroleptic-induced tardive dyskinesia were given 12 mg/day of ondansetron for 12 weeks in an open-label study. Administration of ondansetron resulted in a statistically significant improvement in tardive dyskinesia and psychotic symptoms. Likewise, co-administration of ondansetron with levodopa was effective in attenuating dyskinesia in the hemi-parkinsonian rat model attesting to the anti-dyskinetic efficacy of 5-HT3 antagonists. Ondansetron has been also shown to improve cerebellar tremors.
Bulimia nervosa
Ondansetron has shown efficacy for bulimia in three small trials.Reference Erzegovesi and Bellodi 5 Ondansetron produced improvements in several parameters of bulimic activity; weekly binge episodes decreased; ondansetron also led to a greater increase in normal meals ingested; and the time occupied by bulimic behavior decreased as well. It was hypothesized that ondansetron acts on vagal 5-HT3 receptors to normalize the dysfunctional pattern of meal termination and satiety resulting from aberrant vagal serotonergic neural activity in bulimic patients.
Schizophrenia
In a systematic review and meta-analysis, ondansetron adjuventia was demonstrated to be safe and efficacious in improving negative symptom domain, including EPS, and general psychopathology. Evidence is conflicting regarding the improvement of cognitive domain.
Parkinson’s disease psychosis
A systematic review identified four open-label trials of ondansetron for psychosis associated with Parkinson’s disease. All trials showed improvements in visual hallucinations and paranoid ideations in most patients, as well as a modest improvement in functioning, but no evidence of cognitive improvement.
Alzheimer’s disease
A preclinical evidence suggests ondansetron could be a potential therapeutic drug for alzheimer’s diseaase (AD) by modulating ApoE metabolism through the LXR-ABCA1 pathway. However, a multicenter, double-blind, placebo-controlled clinical trial to assess the efficacy and safety of ondansetron (20 and 100 μg/day) in treating cognitive decline in 185 patients with Alzheimer disease, failed to demonstrate any significant cognitive improvement. Interestingly, in an RCCT, the postoperative ondansetron administration seemed to protect and might improve the cognitive function in patients undergoing surgery under general anesthesia. Ondansetron also seems to release analgesic effects.
Panic disorder
In a randomized, double-blind, placebo-controlled design, ondansetron 1 mg bid showed a significant reduction in anxiety symptoms (mean Hamilton anxiety rating scale (HARS) score) compared to placebo. Constipation was a common side effect. This multicenter study of 54 participants taking ondansetron shows promise.
Tinnitus
Ondansetron also blocks the alpha-9 and alpha-10 receptors, and it has been hypothesized that this action increases cochlear sensitivity to external stimuli and may alleviate tinnitus as shown in one RCT.
It should be noted that level of evidence supporting the use of ondansetron in all these aforementioned psychiatric indications is highly variable and, hence, sound clinical judgment, manipulating first all other viable treatment options at hand, would dictate its judicious and proper use and placement in real-life psychiatric practice and psychopharmacotherapy algorithms.
Disclosure
The authors do not have any competing interests or financial affiliations.



