Introduction
Migraine is recognized as a complex disorder with severe, disabling, and neurological symptoms that place a major burden on patients, society and the healthcare system.Reference Bonafede, Sapra, Shah, Tepper, Cappell and Desai 1 – Reference Stovner, Nichols and Steiner 3 Migraine affects about 12% of the general population, and it is typically characterized by attacks of throbbing headache that are often aggravated by activity and accompanied by nausea, vomiting, sensitivity to light, and/or sound. 4 – Reference Woldeamanuel and Cowan 6 The effect of migraine extends beyond the physical pain as it impacts significant psychosocial aspects of an individual’s life. Although the condition is considered to be affected by multiple endogenous (eg, genetic, epigenetic, neurochemical, neuroendocrine, and neuroanatomical) and exogenous (eg, societal, rearing, environmental, and nutritional) underpinnings,Reference Andreou and Edvinsson 7 – Reference Guidetti, Faedda and Siniatchkin 10 there is currently no framework available for incorporating all of these factors implicated in migraine. While it is well documented that migraine is not static since internal and external modulatory factors, such as hormonal changes, phases of life, stress, and sleep, can influence the level of excitability of the migraine brain, disentangling the contribution of these underpinnings can be a daunting task given that they closely interact.
One way to uncover the complex interaction among these underpinnings is along the time axis of the transient (ie, state) vs enduring (ie, trait) patterns and their potential interactions within the “migraine phenotype.” The innate structural and biochemical makeup of the brain defines its functions and susceptibilities. When neural processing is dysfunctional, neural responses may evolve into a specific behavioral or phenotypic format (eg, increased susceptibility to headaches).Reference Lai, Protsenko, Cheng, Loggia, Coppola and Chen 11 , Reference Schwartz and Rauch 12 Thus, neural processing in the disease state (eg, migraine context), while ongoing, may fluctuate within and between normal (ie, resistant brain state) and abnormal limits (ie, susceptible brain state).Reference Garner and Mayford 13 Our aim is to give a migraine model in the context of how different traits and states could work together to determine this disorder. Figure 1 summarizes the proposed interactions and the overall theme of the paper.
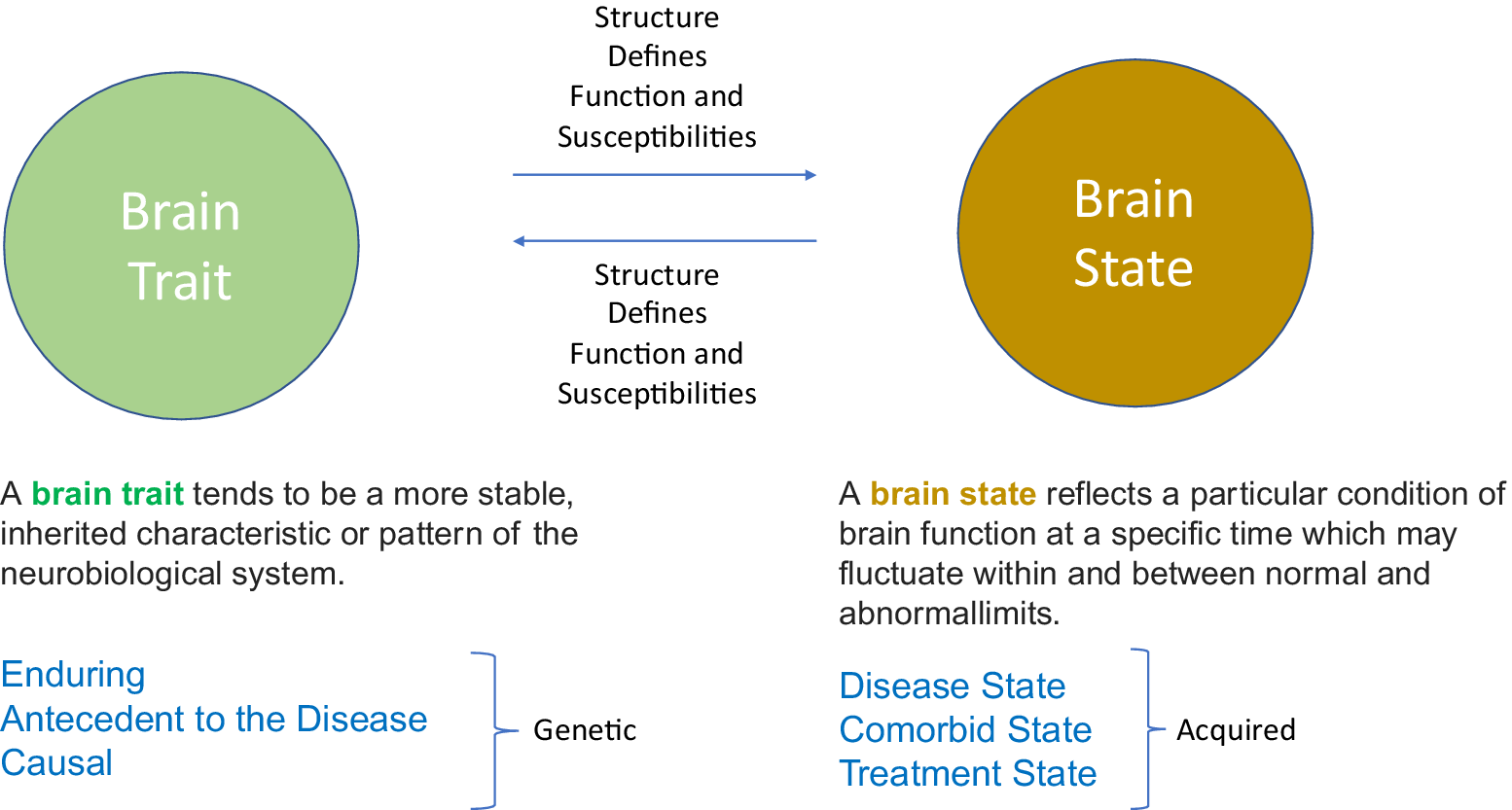
Figure 1. The basic concept. Using the two domains of brain trait and brain state provides a unique framework for understanding the role of neurobiological system in the context of internal and external processes in migraine.
Migraine could be a perfect model to dissect the role of neural circuit function/dysfunction (trait) in the context of alterations of the internal and external environment (state): While there are certain trait components to migraine, current states are altered by different events which may directly lead to migraine attacks or reduce the attack threshold by making the brain more responsive to trigger factors.Reference Eising, Datson, van den Maagdenberg and Ferrari 14 – Reference Wessman, Terwindt, Kaunisto, Palotie and Ophoff 16 Migraine is considered as a chronic disorder with trait-like features interspersed with acute and temporary episodes, signs, and symptoms.Reference Burstein, Noseda and Borsook 17 , Reference Puledda, Messina and Goadsby 18 Defining migraine as a chronic disease also implicates that the interictal migraine state is not a “healthy” brain state. Increasing evidence suggests that brain networks involved in the processing of pain, emotions, and sensory stimuli show stronger functional connectivity and hyperexcitability even between the attacks which supports the notion that there are enduring, underlying traits that are involved in migraine.Reference Borsook and Dodick 19 , Reference Sprenger and Borsook 20 In addition, while most migraine patients have a polygenetic underpinning,Reference Sutherland, Albury and Griffiths 21 migraine like-symptoms can also occur following head trauma,Reference Capi, Pomes, Andolina, Curto, Martelletti and Lionetto 22 , Reference Lew, Lin, Fuh, Wang, Clark and Walker 23 seizures,Reference Nye and Thadani 24 or medication overuse,Reference Kristoffersen and Lundqvist 25 as examples, in individuals who have not had a history of migraine attacks. These brains were affected by physical impact or stress leading to neural dysfunction. This speaks to the potential interplay of state and trait, since the headache initially occurs after an event (eg, brain injury), but the following structural changes are connected to the presence of recurrent migraine states.Reference Obermann, Nebel and Schumann 26 In line with this, the phenomenon of migraine progression is also an example of state–trait interplay where the overriding state drives an underlying trait (functional, structural, and chemical) leading to disease transformation or chronification.Reference Aurora and Brin 27 Studies showing brain alterations that are connected to the migraine attack frequency and disease duration (number of years with migraine) also strengthen this assumption.Reference Coppola, Petolicchio and Di Renzo 28 , Reference Liu, Chou and Lee 29
Here, we use this “migraine-model” to evaluate underpinnings of the state–trait contributions to the manifestation of the disease in three major sections: (a) migraine traits (MTs)—in the context of genetics and disease expression; (b) migraine states (MSs)—in terms of entropy vs negentropy (the reverse of entropy); and (c) migraine interactive states (MIS).
Migraine Traits (MTs)
We suggest a classification of primary and secondary MTs. Primary MTs (PMTs) are those brain traits specific to a migraine process that is inherent in an individual’s genetic makeup/biology, perhaps termed “the migraine brain.” Secondary MTs (SMTs) are those inherited conditions such as behavioral (eg, increased risk of depression and anxiety influenced by shared genetic factors) or structural (eg, ectopic vessel) that drive the PMTs.Reference Schur, Noonan, Buchwald, Goldberg and Afari 30 – Reference Zhang, Shao, Jiang, Tsai and Liu 32 Sensitivity to stress attack,Reference Huber and Henrich 33 vulnerability to negative affectReference Silberstein, Lipton and Migraine 34 or underlying autonomic dysfunctionReference Gazerani and Cairns 35 , Reference Ozer 36 may be SMTs that contribute to the lowering of the threshold for a migraine in patients with a PMT profile. However, secondary traits may also provide protection, for example, trait optimism has a protective effect against anxiety.Reference Wang, Zhao and Cheng 37 It should be also noted that these SMTs, such as the sensitivity to stress, could influence the onset and maintenance of migraine states (MSs) (see below), but the recurring migraine attacks may be also related to experiencing more negative emotions and stress indicating state and trait interaction.Reference Mose, Pedersen, Jensen and Gram 38 Some traits could change with progression or remission of a disease or other biological factors such as age and sex.Reference Smitherman and Ward 39 In addition, they may be related to another underlying disease (eg, attention deficit hyperactivity disorder, major depressive disorder [MDD], or Tourette syndromeReference Anttila and Bulik-Sullivan 40 ). It is worth mentioning that such conditions may not be traits per say, but contributing factors. The psychiatric co-morbidities and how stress and migraine are related also support an interaction between somatic and psychological factors in migraine (especially in younger children).Reference Guidetti, Faedda and Siniatchkin 10 As such, migraine could be a dysfunctional adaptation to predispositions (genetic) as well as endogenous and exogenous events, where somatic complaints and emotional distress may be strongly related.
Genome-wide association studies (GWAS) have identified genetic loci involved with MTs. However, it is unclear what the contributions of genetic differences are as they relate to disease expression or load. As an example, genetic load appears to be higher in hemiplegic migraine and migraine with aura compared to without aura supporting the view that migraine could be a spectrum disorder.Reference de Boer, Terwindt and van den Maagdenberg 41 Furthermore, such traits may also relate to reactivity of tissue (nerves, glia cells, and vessels) to processes such as cytokine responsivity.Reference Hauberg, Zhang and Giambartolomei 42 Recent GWAS studies in patients with migraineReference Anttila, Winsvold and Gormley 43 , Reference Gormley, Anttila and Winsvold 44 have identified single nucleotide polymorphisms (SNPs) in target tissue, including blood vessels, that are associated with migraine risk. However, the contribution of variants in SNPs to their migraine susceptibility remains to be defined, independent of or accounting for various MSs. For example, independent of MS, there are overlapping genetic variants (SNPs rs146377178, rs672931, and rs11858956) in migraine and MDD.Reference Yang, Zhao and Boomsma 45 Evaluation of GWAS data with gene expression in human brains has implicated some neurochemical pathways involving the cortex, cerebellum, and subcortical areas, suggesting a connection between neurochemical changes and migraine pathophysiology.Reference Eising, de Leeuw and Min 46 However, it is worth noting two major caveats relating to genetically predisposed disease states that could be applied to MTs: (a) that generally disease prediction based on traits is difficultReference Schrodi, Mukherjee and Shan 47 and (b) that interactions with the environment are complex and may alter susceptibility of the migraine phenotype. Penetrance of a disease depends on the proportion of individuals with a genetic trait that actually exhibit the characteristic phenotype. Thus, expression of the disease may relate to the relative environmental load encompassing social, psychological, and physical stressors and the relative reactivity of the individual based on genetic “load”Reference de Boer, van den Maagdenberg and Terwindt 48 previously considered in the domain of “migrainomics.”Reference Nyholt, Borsook and Griffiths 49 Most studies, specifically identifying variants that can contribute to the migraine disorder, have concentrated on the SNPs associated with an increased or decreased risk of migraine that may influence migraine susceptibility but not necessarily lead to migraine onset. That is, genetic research further supports the complexity of migraine, and emphasizes that traits could interact with states.Reference Sutherland, Albury and Griffiths 21
Migraine States (MSs)
A disease state may be defined as a condition that an individual has and which manifests with well-defined signs or symptoms. While the International Headache Society (IHS) has defined various migraine subtypes (http://www.ihs-headache.org/ichd-guidelines), we refer to primary MSs as that related to the headache itself with regard to duration, intensity and whether acute or chronic; whereas secondary MSs as that related to the nonheadache phenomena (eg, depression, autonomic features, tiredness, nausea, vomiting, and sensitivity to light and/or sound) that may be present either in the interictal or ictal state. Migraine symptom variants also include those that may exist alone or in combination outside of or without headache (eg, visual aura, atypical auras, confusion, abdominal pain, cyclic vomiting, vertigo, hemiplegia/hemiparesis [severe or mild loss of strength on one side of the body], and dysarthria [speech problems]). As such, disease states may refer to (a) disease load (ie, remission vs minimal activity or severe activity); (b) temporal process (ie, acute or chronic; intensity, frequency); and (c) treatment responsivity. It usually reflects the clinical evaluation/impression of the individual’s migraine condition. What is more difficult to capture is the undulating nature of the disease state and the contributions of biological and environmental processes. We discuss examples of how brain traits may interact with brain states below.
Notably, MSs may be viewed as unstable brain states. As such they may be considered entropic (more unstable or degree of disorder) or negentropic (more stable or more ordered) conditions. If one considers a stable state such as health, patients with few episodic migraines of low intensity or duration may be viewed mildly entropic whereas patients with severe or chronic migraine may be considered more entropic. Stabilizing factors such as treatment, decreased stress could convert or reverse the entropic state toward the more stable negentropic state. These states and resistance to treatments may be models for overall migraine state. Negentropy has been used to differentiate headache states.Reference Biyouki, Rahati, Laimi, Boostani and Shoeibi 50 Such approaches if reproducible can provide a relative state along the continuum of entropy vs negentropy.
Migraine Interactive States (MIS)
Behaviors are related to the function of the neural networks.Reference Bassett and Bullmore 51 , Reference Mears and Pollard 52 Migraine behaviors can be defined in terms of the headaches and a multitude of other phenomena related to the condition as noted in “MSs” above. Modulation of these behaviors may relate to the state of the brain as well as environmental and multiple other factors at the time. In migraine, there is an abnormal brain function: Structural, functional, and chemical alterations are well documented including changes in gray matter or white mater volumes, functional connectivity alterations (during and between migraine attacks), white matter alterations, infarct-like lesions, as well as dysfunctional energy metabolism and mitochondrial disruption in migraine patients compared to healthy controls.Reference Ashina, Bentivegna, Martelletti and Eikermann-Haerter 53 These brain changes are exacerbated or improved based on relative effects of environmental (eg, socioeconomic status) or psychological (eg, anxiety) conditions. Variations in brain alterations could be also attributed to the different disease states (eg, migraine duration, pain intensity, attack frequency, and treatment responsivity). Brain changes can be detected in the interictal period as well showing slow progression that reaches a threshold in the preictal period beyond which the migraine attack starts.Reference Peng and May 15 , Reference Karsan and Goadsby 54 , Reference May and Burstein 55 Considering different states and traits and their interaction with each other may provide insight into the migraine condition, for example, how different triggers activate migraines in susceptible individuals, or how individuals with an increased risk for migraine do not manifest any symptoms at all. An example of the latter is the use of drugs to activate/provoke headaches (may not be necessarily migraine) in migraine patients but not in healthy individuals.Reference Ashina and Hansen 56
Weight and effect—a model for MIS
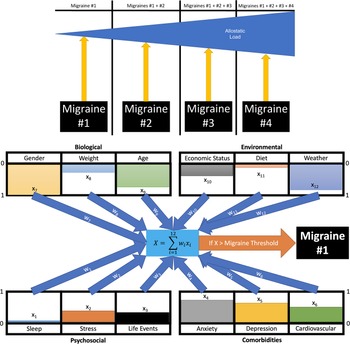
Using the concepts of weight—the value of a component (such as age, sleep, etc.), and effect size—the relative quantitative contribution (magnitude) of that process in the resulting action (migraine), we can model the MIS using machine learning models (Figure 2). For example, deep learning models allow interactions in a matrix using “an exchangeability property.”Reference Hartford, Graham, Leyton-Brown and Ravanbakhsh 57 In this way, the matrix may include biological interactions (eg, sex, weight, sleep patterns, and sensory summation), and emotional interactions (eg, fear, anxiety, depression, and psychological stress). We have these in conjunction with the deep learning model of migraine in the context of interactions of trait and state. A few examples are provided below:

Figure 2. Migraine load and events. Twelve example events are derived from the four conditions that can induce migraines within patients (lower panel). Each event (x 1-x 12) is given a value between 0 and 1 (presented by the color bars) for how strong the state is during the induced event. These are modified by weights (w 1-w 12) between 0 and 1 mimicking genetic propensity for how much variable changes will affect the induced migraine in a particular patient. For example, if stress is shown to have a high correlation with the risk of migraines, then the weight will be closer to 1, while if lack of sleep is unrelated, it will tend toward 0. The summation ∑ 12 i = 1 wixi then provides a final numeric value. If this value is greater than the threshold, then a migraine is induced. Successive migraines over time can build towards an ongoing effect that contributes to an allostatic load that could increase the severity of the pain (upper panel).
Summation of effects—multisensory disarray
Contribution of repeated migraines on altered primary sensory systems has been well documented.Reference Harriott and Schwedt 58 Why some individuals have predominantly or only one sensory system affected (eg, allodynia) remains unclear and may reflect underlying trait or trait penetrance. However, the more primary sensory systems affected, there seems to be a greater disarray of sensory performance. In our recent paper,Reference Hodkinson, Veggeberg and Kucyi 59 we evaluated complexity of the sensory networks as they converge and become functionally coupled in multimodal systems and compared self-reported retrospective migraine symptoms in the same patients, examining the prevalence of different primary sensory symptoms (ie, photophobia, phonophobia, and osmophobia) across the phases of the migraine cycle. The data suggest widespread and persistent disturbances in the perceptions of multiple sensory modalities. Functional magnetic resonance imaging results indicate that these primary sensory areas maintain local functional connectivity but exhibit impaired long-range connections to higher-order association areas (including regions of the default mode and salience network). Such data implicate that trait-dependent migraine load (ie, frequency, duration of disease, intensity, aura, etc.), represented by altered cortico-cortical interactions may be strongly connected to the ongoing disarray of sensory integration. In keeping with this theme, the data seem overwhelming that patients with aura have significantly worse outcomes in terms of brain and behavioral changes.Reference Jürgens, Schulte and May 60 In the latter, migraine with aura was reportedly found to have greater presence of a number of neurologic symptoms and be associated with a higher frequency of autokinesis, metamorphopsia, dyschromatopsia, cinematographic vision, illusionary visual spread, and synesthesia.Reference Jonas and Hibbard 61 Thus, migraine patients with aura seem to have a much severe form of the disease and, as such, the overall migraine load is expected to be exacerbated.
Exacerbation by hormonal environment
Sex makes a difference in migraine. Menarche, menstruation, pregnancy, menopause, and hormonal contraceptives are well known to exacerbate or inhibit migraine.Reference Sacco, Ricci, Degan and Carolei 62 Estrogen is implicated as a major factor in female related migraineReference Chai, Peterlin and Calhoun 63 possibly by moderating the trigeminal neuron function more generally and the intracranial vasodilatory response,Reference Warfvinge, Krause, Maddahi, Edvinsson, Edvinsson and Haanes 64 and cross-sex administration of estrogen in female transgender patients may lead to a worsening of headache.Reference Aloisi, Bachiocco and Costantino 65 Estrogen receptors are richly expressed in areas of the central nervous system and the trigeminovascular system that are known to be involved in migraine pathophysiology.Reference Warfvinge, Krause, Maddahi, Edvinsson, Edvinsson and Haanes 64 In children, the prevalence of migraine in males and females is similar until puberty,Reference Böttcher, Kyprianou and Lechner 66 , Reference Eidlitz-Markus and Zeharia 67 but after around the age of 11 years females are predominantly affected.Reference Wilcox, Ludwick, Lebel and Borsook 68 On a similar note, its prevalence increases at puberty in girls and decreases in postmenopausal women.Reference Lipton, Bigal and Diamond 5 We have previously reviewed sex differences in migraine that summarizes some of these issues.Reference Borsook, Erpelding and Lebel 69 Thus, a given trait (sex) is influenced by an exogenous modulator (estrogen), providing an example of the trait–state interaction.
Circadian disruption
Altered sleep (duration, sleep–wake cycles) may trigger migraine attacks.Reference Lin, Lin and Lee 70 Patients with migraine display lower sleep quality and have more difficulty to overcome alterations in sleep–wake cycle. Furthermore, days early in the week, especially among teenagers, are the worst days for migraine attacks.Reference Winner, Rothner, Putnam and Asgharnejad 71 Rapid large changes in schedule including travel to different time zones and shift work may also contribute to a lower threshold for a migraine attack.Reference Sandoe, Sasikumar, Lay and Lawler 72 The suprachiasmatic nucleus is suggested to be the initial site of migraine attacks, and sleep rhythms are driven by this region, thus, a major biological disruption, presumably acting through the circadian pacemaker of the brain, can exacerbate migraine and presumably affect when the migraine typically strikes (eg, prevalent morning or evening onset).Reference Baksa, Gecse and Kumar 73 , Reference Burish, Chen and Yoo 74 Such oscillatory changes may also reflect changes in brain susceptibility.Reference Borsook and Burstein 75 Sensitivity to triggering factors or the way nociceptive information is perceived may be altered by disrupted homeostatic regulation, but the vulnerability to different stimuli could also challenge the brain’s homeostasis. Also, it still needs to be determined if these atypical homeostatic events are conditions enhancing migraine attacks or represent migraine symptoms or relate to both.Reference Goadsby, Holland, Martins-Oliveira, Hoffmann, Schankin and Akerman 76
Psychiatric/psychological co-morbidity
A strong genetic association between anxiety trait and migraine trait has been reported,Reference Stam, de Vries and Janssens 31 , Reference Ligthart, Nyholt, Penninx and Boomsma 77 and as such anxiety is more common in individuals who have migraine.Reference Senaratne, Van Ameringen, Mancini, Patterson and Bennett 78 Anxiety is a useful model since there may be an underlying anxiety trait, besides the expectancy related to migraine attacks, which may itself induce an anxiety state as a transitory emotional state or condition that can vary in intensity and fluctuate based on the situation, and characterized by feelings of apprehension, tension, and physiological symptoms (eg, increased heart rate or respiration).Reference Spielberger 79 However, interindividual differences relating to frequency and severity are present. While trait anxiety is described as a rather stable and generalized vulnerability to experience anxious states, state anxiety is likely influenced by the interaction of trait anxiety and situational factors.Reference Endler and Kocovski 80 , Reference Shedletsky and Endler 81 Individuals with high trait anxiety are more likely to display hyper-responsivity to aversive events or situations, which in turn activates state anxiety. This state can be also determined by attentional processing and behavioral characteristics that contribute to the impact of long-term trait vulnerabilities.Reference Nelson, Purdon, Quigley, Carriere and Smilek 82 , Reference Robinson, Krimsky and Grillon 83 The way these factors interact on a variety of timescales can also be applied to migraine, where MTs represent a generalized and enduring predisposition to respond to different internal and external events in a relatively consistent manner, and can explain individual differences in the duration, frequency, and intensity of MSs. Thus, the anxiety-migraine continuum could be a useful model for evaluating disease strength and disease load interactions. Similarly, shared genetic and environmental factors between migraine and other psychiatric conditions such as depression and panic disorder, which seem to have a bidirectional association with migraine, may also yield useful models.Reference Dresler, Caratozzolo and Guldolf 84 , Reference Yang, Zhao, Heath, Madden, Martin and Nyholt 85 Of interest, the presence of psychiatric co-morbidities (anxiety and depression) has been related to the severity of migraine symptoms as well.Reference Antonaci, Nappi, Galli, Manzoni, Calabresi and Costa 86 , Reference Lipton, Seng and Chu 87
Modeling trait–state conditions
Determinants of migraine responsivity are difficult to define. Figure 2 shows a model of four processes that may enhance or diminish migraine responsivity based on an individual’s trait and state. In addition, a summarized description of the most common conditions that could alter migraine is provided by Table 1. Again, it is worth mentioning that some of these conditions (disease comorbidities) may not only be aggravating or mitigating events for migraine but could originate from the same genetic source. In other words, this does not inevitably indicate that patients who have more comorbidities also show enhanced migraine responsivity. As an example, some patients may have both depression and migraine, but there are also patients who are depressed but do not have any migraine attacks.
Table 1. Summary of the Most Common Conditions that may Enhance or Diminish Migraine Responsivity
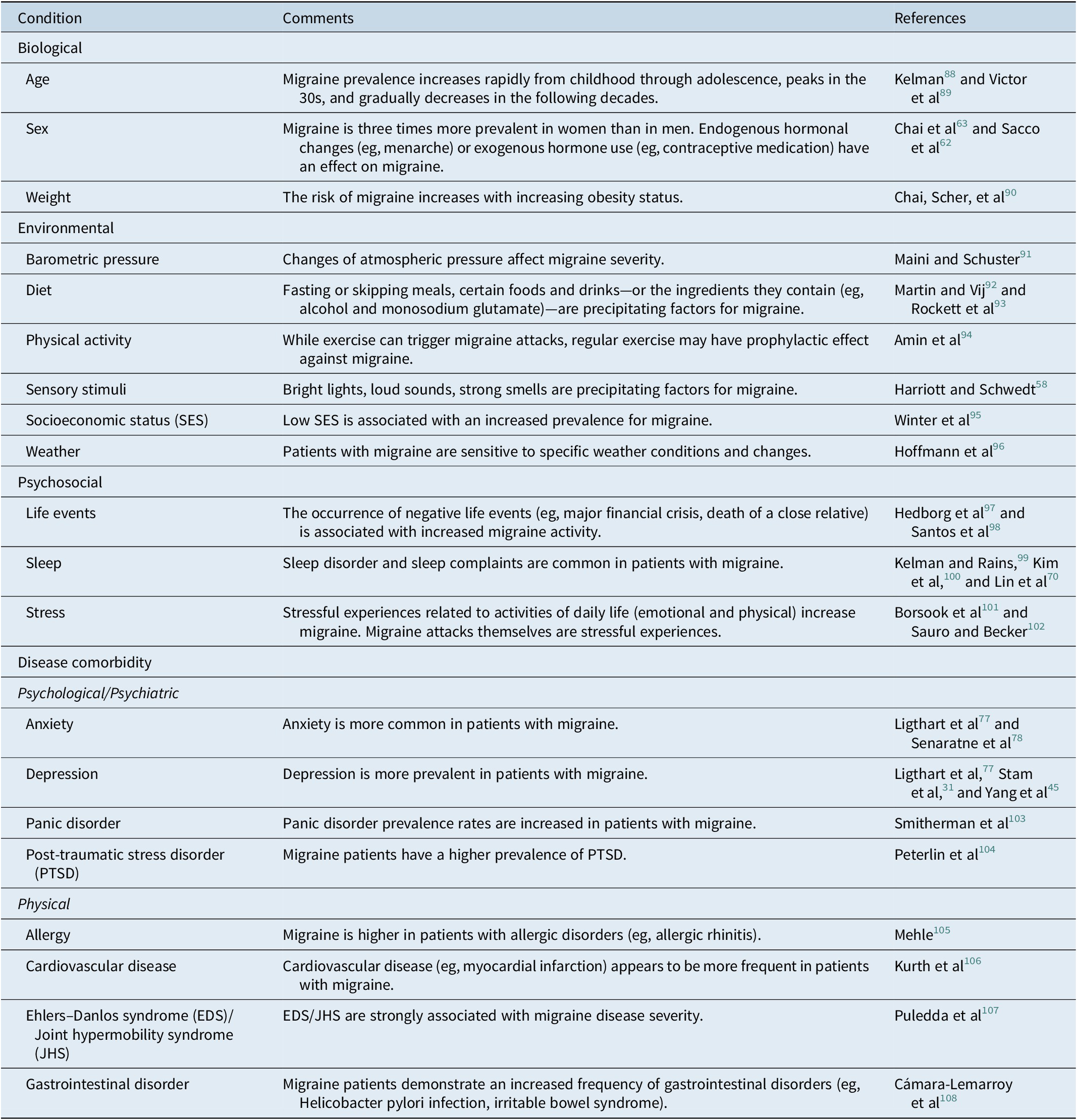
These conditions were selected based on suggestions of the literature as being significant contributors to migraines.
Variability across Individuals
Interindividual differences relating to migraine characteristics/presentation should be also taken into account.Reference Lipton, Fanning and Buse 109 For example, in some patients, nausea and vomiting are more likely to be observed, whereas other patients show allodynia symptoms. That is, the overall load of migraine disease and the specific events that may alter migraine responsivity (see Figure 2) could differ from patient to patient. Identifying migraine subgroups based on individual differences in migraine manifestation could help understand the role of the disease state in relation to the external and internal environment. Also, individuals with migraine do not form a homogeneous group in terms of underlying biological predispositions. What these patients share is the experienced MSs, and more frequently recurring states could indicate a triggering environment exposure, a stronger biological risk factor to experience these states, or complex interactions between the two.
Clinical Implications
In patients care, focusing on the potential interplay of migraine-specific traits and current states could be essential in a perspective of precision medicine approach, facilitating risk evaluation and tailored treatment of migraine. This personalized approach accounts for each patient’s genetic, environmental, and lifestyle factors to develop intervention and prevention that are adapted to individuals or groups.Reference Maier 110 , 111 This could include identifying individual risk factors for developing migraine disease (traits), and predicting response to different pharmacological treatments or the likelihood for drug-related adverse events (states). While the majority of treatment options have been nonspecific to migraine and restricted by adverse events and medical comorbidities, the development of novel medications expands the range of options for both acute and preventive treatment of migraine (including gepants, and anti-calcitonin gene-related peptide monoclonal antibodies).Reference Ceriani, Wilhour and Silberstein 112 , Reference Do, Guo and Ashina 113 Although medication is the mainstay of migraine treatments, patients experiencing migraines can benefit from nonpharmacological approaches as well, such as cognitive behavioral therapy and mindfulness-based interventions, which can decrease the physical symptoms of headache (migraines states) and enhance psychological well-being and migraine disability.Reference Harris, Loveman, Clegg, Easton and Berry 114 – Reference Wells, Seng and Edwards 116 Migraine treatments should be optimized based on the presence of psychiatric co-morbidities, and the potential bidirectional relation of migraine with depression and panic disorders makes this population a promising candidate for synergistic combination therapy.Reference Dresler, Caratozzolo and Guldolf 84 Monitoring and evaluating MTs and states can also help identify treatment responders and nonresponders.
Conclusion
Consideration of migraine as a brain state–trait interaction in response to environmental and/or endogenous events is a view that has scientific support in the literature. Based on the framework outline here, contribution to patient care would be metrics to evaluate a migraine patient’s underlying trait/genetic status and to be able to define the importance of their current brain state based on physiological, psychological, and environmental measures. As such, more specific therapeutic approach may be considered in the context of disease susceptibility and progression risk. One approach to this is a personal technology/system that continually monitors some of these interoceptive and exteroceptive measures consistent with the rapid evolution in the development of both hardware and software. For example, and consistent with this notion is the use of watch systems for the prediction of migraine attacks.Reference Houtveen and Sorbi 117 , Reference Sano, Taylor and McHill 118 Given the multiple behavioral and physiological changes in migraine, the use of multiple low-dose therapies for prevention of migraine attacks would seem to make sense, since there are multiple receptor targets within many migraine-related tissues derived from different brain structures. Also, measuring physiological and behavioral data (eg, skin conductance and sleep activity) could give further insight into the different elements affecting the migraine load.
Funding
This work was supported by the National Institutes of Health (R01NS056195 and K24NS064050 to DB).
Disclosures
The authors do not have anything to disclose.