Introduction
Functional impairment is a key consequence of major depressive disorder (MDD).1, Reference IsHak, James and Mirocha2 Multiple domains of functioning are typically impaired in patients with MDD, particularly their ability to work and work productivity.Reference Beck, Crain and Solberg3–Reference Uribe, Pinto, Vecino-Ortiz, Gómez-Restrepo and Rondón7 Depression has a considerable impact in the workplace worldwide.Reference Evans-Lacko and Knapp8 Of ∼1.3 million Canadians aged 15–65 years who experienced a depressive episode in 2012, over 1 million were employed.Reference Stonebridge and Sutherland9 Of these, only 17% were reported to be fully functioning at work; 23% were unable to work due to their depression, 20% worked part-time due to their depression, and 40% worked full-time but with reduced functioning.
The Canadian Network for Mood and Anxiety Treatments (CANMAT) guidelines for the management of MDD state that recovery from depression involves both relief of symptoms and improvement of functioning.Reference Lam, McIntosh and Wang10 MDD is a multidimensional disease characterized by emotional, physical, and cognitive symptoms, all of which may require assessment and treatment to achieve functional recovery.Reference Lam, McIntosh and Wang10–Reference Sheehan, Nakagome, Asami, Pappadoulous and Boucher14 Systematic reviews have shown that improvement in mood symptoms is only modestly correlated with functional outcomes in MDD.Reference McIntyre, Lee and Mansur13, Reference Buist-Bouwman, Ormel and de Graaf15–Reference Iosifescu17 Functional impairment can persist in patients with MDD even after remission of mood symptoms,Reference IsHak, James and Mirocha2, Reference Kennedy, Foy, Sherazi, McDonough and McKeon18, Reference Trivedi, Morris and Wisniewski19 and residual functional impairment has been associated with an increased risk of relapse and recurrence of depression.Reference Solomon, Leon and Endicott20, Reference Hardeveld, Spijker, De Graaf, Nolen and Beekman21
The clinical relevance of cognitive symptoms, including disturbances in attention, memory, processing speed, and executive functioning, and their role in work-related disability is well documented in MDD.Reference Lee, Hermens, Porter and Redoblado-Hodge16, Reference Hasselbalch, Knorr and Kessing22–Reference Knight and Baune27 Compared with depression severity, cognitive symptoms have been reported to account for greater impairment in workplace functioning in patients with MDD.Reference McIntyre, Soczynska and Woldeyohannes28 Treatment of cognitive symptoms may hold the key to achieving functional recovery in MDD; however, the relationship between cognitive symptoms and functional impairment in MDD is not well understood.
Vortioxetine is a multimodal antidepressant approved for the treatment of MDD in adults, which acts as an inhibitor of the serotonin transporter as well as modulating the activity of multiple serotonin receptor subtypes.Reference Garnock-Jones29, Reference Sanchez, Asin and Artigas30 Vortioxetine has been shown to be effective not only for the treatment of MDD,Reference Thase, Mahableshwarkar, Dragheim, Loft and Vieta31, Reference Vieta, Loft and Florea32 but also to improve cognitive symptoms in patients with depression.Reference McIntyre, Lophaven and Olsen33–Reference Baune, Sluth and Olsen38 A recent meta-analysis showed that vortioxetine also demonstrates efficacy in improving overall functioning and functional remission, as assessed by the Sheehan Disability Scale (SDS), in adults with MDD.Reference Florea, Loft and Danchenko39
Assessment in Work productivity and the Relationship with Cognitive symptoms (AtWoRC) is an interventional, open-label, real-world study undertaken to examine the association between cognitive symptoms and workplace productivity in working Canadian patients with MDD treated with vortioxetine. The primary analysis of this study showed a statistically significant association between improvements in cognitive symptoms in patients with MDD and workplace productivity after 12 weeks of vortioxetine treatment.Reference Chokka, Bougie, Rampakakis and Proulx40 Patients with MDD generally require long-term treatment; current CANMAT guidelines recommend antidepressant treatment continuing for at least 6 months after achieving symptomatic remission and for 2 years or longer in patients with risk factors for recurrence.Reference Lam, McIntosh and Wang10 However, only limited data are available concerning the long-term real-world effectiveness of antidepressants. This article presents the analysis of the AtWoRC study after 52 weeks of vortioxetine treatment in clinical practice, together with structural equations model (SEM) analyses undertaken to further assess causal relationships between long-term changes in measures of cognition and functioning in working patients with MDD.
Methods
Study design
AtWoRC is an interventional, open-label study conducted at 26 sites across Canada, mainly in primary care settings (ClinicalTrials.gov identifier: NCT02332954). The study design and inclusion/exclusion criteria have been reported in detail previously.Reference Chokka, Bougie, Rampakakis and Proulx40 Briefly, eligible patients were aged 18–65 years; were in employment (working ≥20 hours/week) or enrolled in full-time post-secondary studies or vocational training; had a current diagnosis of MDD according to Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5™) criteria1 and an investigator-confirmed current major depressive episode of at least 3 months’ duration; had a baseline Quick Inventory of Depressive Symptomatology–Self-Report (QIDS-SR) score ≥15 and a baseline 20-item Perceived Deficits Questionnaire–Depression (PDQ-D-20) score ≥30; and had not previously received vortioxetine. Exclusion criteria included the following: Digit Symbol Substitution Test (DSST) score >69 at screening/baseline; current diagnosis or history of mania or hypomania, schizophrenia, or any other psychotic disorder (including MDD with psychotic features), personality disorder, attention-deficit hyperactivity disorder, mental retardation, pervasive development disorder, organic mental disorders, or mental disorder due to a general medical condition (DSM-5 criteria); physical, cognitive, or language impairment of such severity as to adversely affect the validity of the data derived from the patient-reported outcomes; current depressive symptoms considered to have been resistant to 2 adequate antidepressant treatments of at least 6 weeks' duration, each at the maximum recommended dose according to Canadian labeling; and previous exposure to vortioxetine.
The study design is shown in Figure 1. All patients received oral vortioxetine 10–20 mg daily (Trintellix®, Lundbeck) and were assessed regularly at visits that emulated a naturalistic, real-life setting as closely as possible. Patients were stratified according to whether vortioxetine was their first treatment for the current depressive episode or they were switching to vortioxetine due to an inadequate response to antidepressant treatment of the current episode. The study duration was 52 weeks, with a safety follow-up visit at week 56. Patients were assessed at visits at baseline and weeks 4, 8, 12, 26, 39, and 52.

FIGURE 1. Design of the AtWoRC study. MDE, major depressive episode (current episode); W, week.
Ethical approval was obtained from the necessary committees for each study site, and all patients provided written informed consent for participation.
Study assessments
The assessment tools used in this study are described in detail in Supplementary Table 1, available online. In brief, depression severity was assessed by patients using the QIDS-SR and by clinicians using the Clinical Global Impressions–Severity and –Improvement scales (CGI-S and CGI-I, respectively). Treatment response was defined as a change in QIDS-SR of ≥50% from baseline. Remission was defined as a QIDS-SR total score ≤5. Cognitive symptoms and performance were assessed by the PDQ-D-20 and the DSST. Functioning and work productivity were assessed by the Work Limitations Questionnaire (WLQ) productivity loss, the SDS, the Work Productivity and Activity Impairment (WPAI) questionnaire, and the 12-item World Health Organization Disability Assessment Schedule 2.0 (WHODAS). Anxiety symptoms were assessed using the Generalized Anxiety Disorder 7-item questionnaire (GAD-7). Safety, tolerability (reporting of adverse events [AEs]), and rate of treatment discontinuation were also assessed.
Statistical analysis
Sample size calculations have been described in detail previously.Reference Chokka, Bougie, Rampakakis and Proulx40 The population for analysis comprised all patients who met the study inclusion criteria and received at least 1 dose of vortioxetine with a valid baseline assessment and at least 1 complete post-baseline visit (full analysis set). Safety and tolerability were evaluated in all enrolled patients who received at least 1 dose of vortioxetine. All efficacy analyses were conducted in the overall population (full analysis set), and for the first-treatment and switch patient groups.
The primary study endpoint was the partial correlation between changes in PDQ-D-20 and WLQ productivity loss scores at week 12; this was also assessed at week 52. The correlation between the change from baseline to weeks 12 and 52 in PDQ-D-20 and WLQ productivity loss scores was described by the partial Pearson’s correlation coefficient adjusted for age, sex, baseline PDQ-D-20, baseline WLQ productivity loss, disease duration, and baseline depression severity (baseline QIDS-SR and CGI-S). Secondary endpoints included change from baseline to weeks 12 and 52 in disease severity (QIDS-SR, CGI-S, and CGI-I), cognitive symptoms and performance (PDQ-D-20 and DSST), work productivity (WLQ productivity loss and WPAI overall impairment), functioning (SDS and WHODAS), and symptoms of anxiety (GAD-7). Rates of treatment response and remission were also calculated at weeks 12 and 52. For all secondary endpoints, Student’s t tests were performed to assess change from baseline and compare between-group differences.
To assess temporal dependence (ie, causality) between changes from baseline in patient-reported cognitive symptoms, functioning, and overall depressive symptomatology, one-lag SEM analyses were performed, which allowed 4 outcome measures to be predicted by the scores of each outcome at the immediately previous visit (PDQ-D-20, DSST, QIDS-SR, with SDS/WLQ productivity loss in 2 separate models). Standardized regression coefficients (SRC) from the SEM were used to evaluate the strength of each relationship over time, with an SRC having an absolute value ≥0.2 considered indicative of robust associations. The 2 models were estimated with the full information maximum likelihood estimator on the full analysis set using the statistical software SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). The full information maximum likelihood estimator produces unbiased estimates in the event of missing data, under the assumption that the data are missing at random. For model evaluation, the root mean square error of approximation and comparative fit index were used (good fit generally accepted as <0.08 and >0.90, respectively).Reference Mueller, Hancock and Osborne41, Reference Kline42 A similar modeling approach was recently used to explore causality between cognitive symptoms, depression severity, and functioning in a longitudinal observational study in European patients with MDD.Reference Larsen, Haro, Saragoussi and Hammer-Helmich43
Results
Study population
The first patient was enrolled in February 2015, and the last patient completed the study in July 2017. Patient disposition is shown in Supplementary Figure 1, available online. A total of 219 patients were enrolled in the study and received at least 1 dose of vortioxetine (107 first treatment, 112 switch). In all, 199 patients attended at least 1 post-baseline visit and were included in the full analysis set (first treatment n = 97, switch n = 102). The mean (SD) daily dose of vortioxetine at week 52 was 15.2 (5.1) mg.
Baseline patient demographics and clinical characteristics are shown in Table 1. Switch patients were significantly older than first-treatment patients (42.6 versus 38.9 years; p = 0.030), and had a longer time since first diagnosis of MDD (11.0 versus 5.6 years; p < 0.001). At baseline, patients in both groups were acutely depressed, with “severe” scores for cognitive symptoms (PDQ-D-20), overall depressive symptoms (QIDS-SR), anxiety symptoms (GAD-7), and functional impairment (SDS). First-treatment patients had more severe anxiety than switch patients (p = 0.029).
TABLE 1. Baseline patient demographics, employment status, and clinical characteristics*
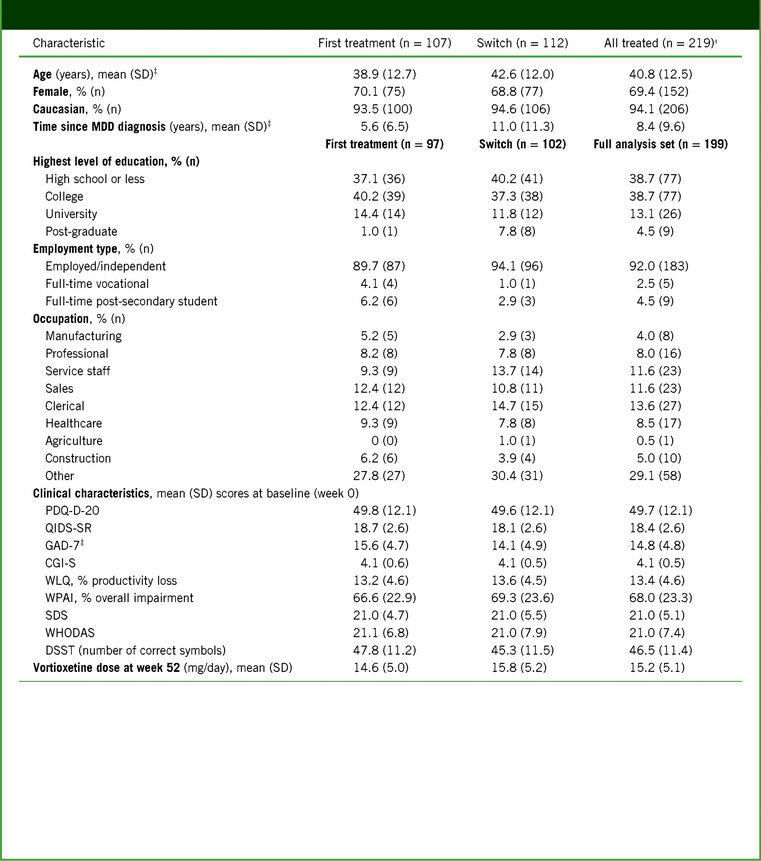
Notes: *For patient demographics, all treated patients were assessed. For employment status and clinical characteristics, patients in the full analysis set were assessed.
† Data for 3 patients were unavailable and therefore not reported at the time of the 12-week primary analysis; information was available at the time of this analysis at week 52 and is included here.
‡ Significantly different between groups (p < 0.05).
CGI-S, Clinical Global Impression–Severity; DSST, Digit Symbol Substitution Test; GAD-7, 7-item Generalized Anxiety Disorder Scale; MDD, Major Depressive Disorder; PDQ-D-20, 20-item Perceived Deficits Questionnaire–Depression; QIDS-SR, Quick Inventory of Depressive Symptomatology–Self-Report; SD, standard deviation; SDS, Sheehan Disability Scale; WHODAS, 12-item World Health Organization Disability Assessment Schedule 2.0; WLQ, Work Limitations Questionnaire; WPAI, Work Productivity and Activity Impairment.
Long-term treatment outcomes
Significant improvements in disease severity (QIDS-SR and CGI-S), cognitive symptoms and objective cognitive performance (PDQ-D-20 and DSST), anxiety symptoms (GAD-7), work productivity (WLQ productivity loss and WPAI overall impairment), and functioning (SDS and WHODAS) were observed over the 52 weeks of vortioxetine treatment (p < 0.001 [paired t test] versus baseline for all outcomes at week 52; Figure 2 and Table 2). No significant differences were reported in mean change from baseline to week 52 between the first-treatment and switch groups for any outcome measure. In all, 77% of patients achieved treatment response (71% in the first-treatment group and 83% in the switch group), and 56% of patients achieved remission (45% in the first-treatment group and 67% in the switch group; p = 0.017) after 52 weeks of vortioxetine treatment (Figure 3). The percentage of patients reporting missed work days due to depression in the past 3 months was reduced, from 55% at baseline to 9% at week 52 (observed cases in the total population). In patients reporting missed work days due to depression, the mean number of work days missed in the past 3 months decreased from 13 days at baseline to 8 days at week 52.

FIGURE 2. Changes in cognitive performance and symptoms, overall depressive symptoms, overall and workplace functioning, and anxiety symptoms over the 52 weeks of vortioxetine treatment. Mean DSST, PDQ-D-20, QIDS-SR, SDS, WLQ productivity loss, and GAD-7 scores over the 52 weeks of follow-up are shown; error bars indicate 95% confidence intervals. Significant improvements (p < 0.001, paired t test) versus baseline were found for all outcomes at week 52. DSST, Digit Symbol Substitution Test; GAD-7, 7-item Generalized Anxiety Disorder Scale; PDQ-D-20, 20-item Perceived Deficits Questionnaire–Depression; QIDS-SR, Quick Inventory of Depressive Symptomatology–Self-Report; SDS, Sheehan Disability Scale; WLQ, Work Limitations Questionnaire.
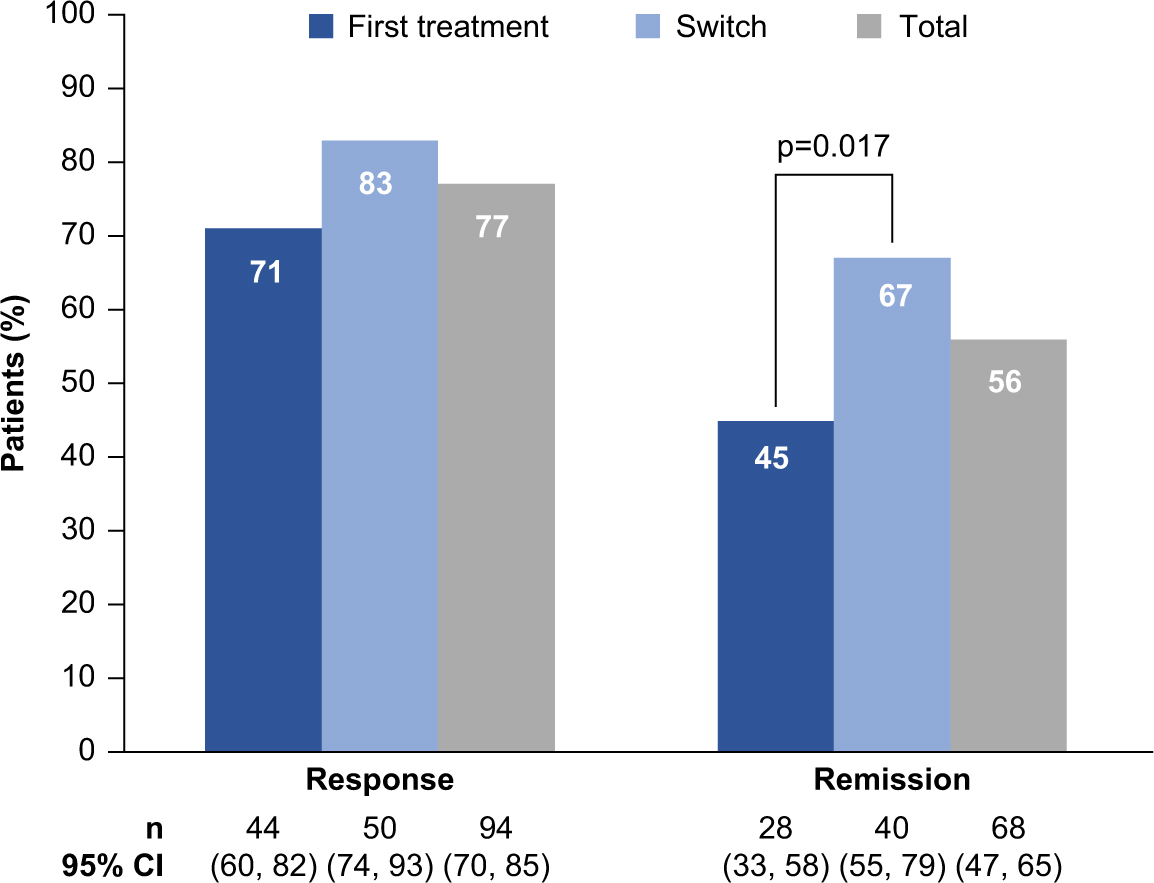
FIGURE 3. Rates of treatment response and remission after 52 weeks of vortioxetine treatment in the overall population and both subgroups (full analysis set, observed cases). CI, confidence interval; n, number of patients who achieved response or remission; p-value refers to the test for difference between the two groups (Fisher’s exact test).
TABLE 2. Change from baseline to week 52 in assessment scores in the full analysis set (observed cases)*
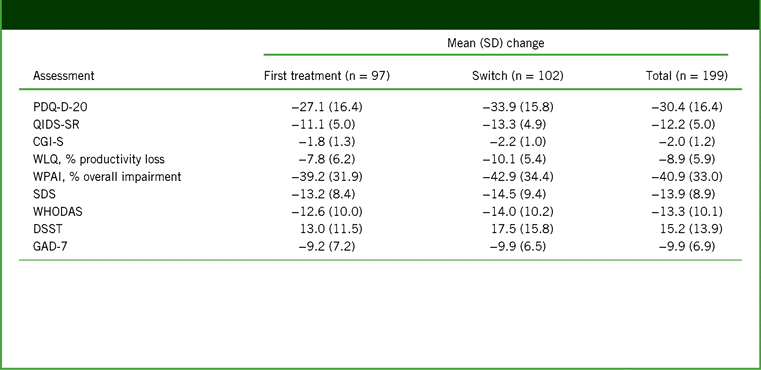
Notes: *All changes are p < 0.001 (paired t test) versus baseline.
CGI-S, Clinical Global Impression–Severity; DSST, Digit Symbol Substitution Test; GAD-7, 7-item Generalized Anxiety Disorder Scale; PDQ-D-20, 20-item Perceived Deficits Questionnaire–Depression; QIDS-SR, Quick Inventory of Depressive Symptomatology–Self-Report; SD, standard deviation; SDS, Sheehan Disability Scale; WHODAS, 12-item World Health Organization Disability Assessment Schedule 2.0; WLQ, Work Limitations Questionnaire; WPAI, Work Productivity and Activity Impairment.
Individual changes from baseline to week 52 in PDQ-D-20 total score and WLQ productivity loss suggested an association between these 2 outcomes (Figure 4). In general, patients who had improved cognitive function following treatment with vortioxetine also had improved workplace productivity. For most outcomes, correlations between changes from baseline to week 52 were highly significant; in particular, significant associations were seen between changes in PDQ-D-20 score and changes in all other outcome measures, including GAD-7 score (Table 3). Reduction in GAD-7 scores was observed at 12 weeks and persisted after 52 weeks of vortioxetine treatment, from “severe” anxiety at baseline (mean score, 14.8; 95% confidence interval [CI], 14.12–15.47) to “mild” anxiety at week 12 (7.7; 95% CI, 6.93–8.56) and week 52 (5.0; 95% CI, 4.04–5.93).
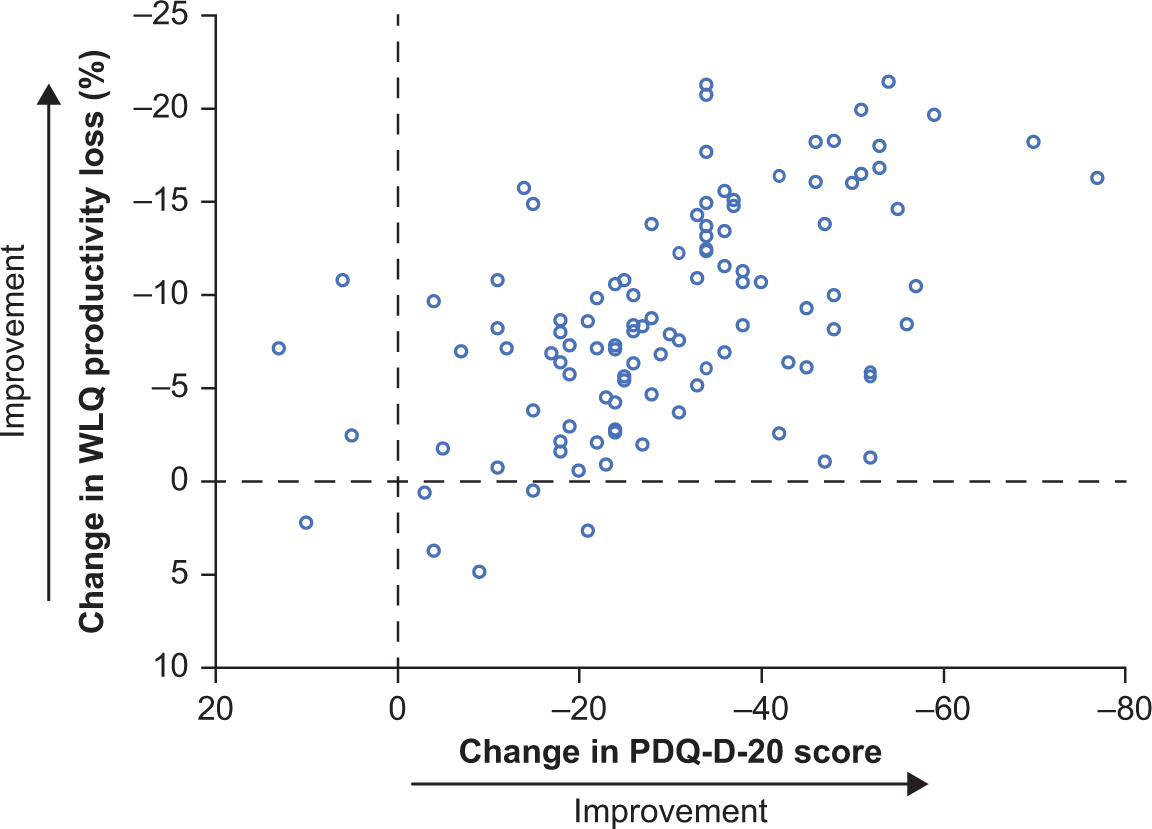
FIGURE 4. Scatter plot of individual changes from baseline to week 52 for PDQ-D20 and WLQ productivity loss scores (full analysis set, observed cases; n = 107). PDQ-D-20, 20-item Perceived Deficits Questionnaire–Depression; WLQ, Work Limitations Questionnaire.
TABLE 3. Pearson correlation coefficients between outcomes for changes from baseline to week 52 (full analysis set)
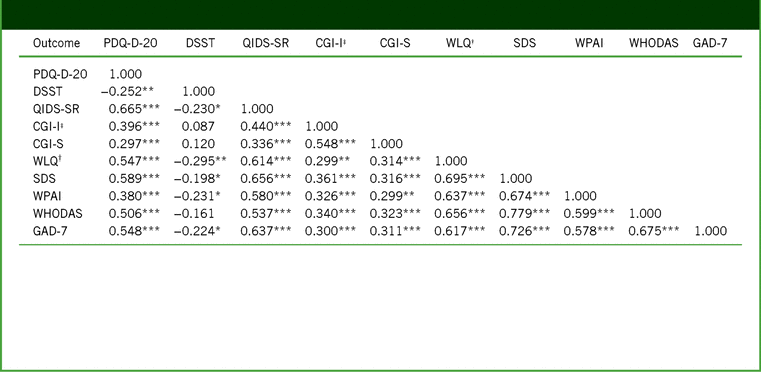
Notes:†WLQ productivity loss, %. ‡CGI-I scores at week 52 used for correlations. ***p ≤ 0.001. **p ≤ 0.01. *p ≤ 0.05.
CGI-I, Clinical Global Impression–Improvement; CGI-S, Clinical Global Impression–Severity; DSST, Digit Symbol Substitution Test; GAD-7, 7-item Generalized Anxiety Disorder Scale; PDQ-D-20, 20-item Perceived Deficits Questionnaire–Depression; QIDS-SR, Quick Inventory of Depressive Symptomatology–Self-Report; SDS, Sheehan Disability Scale; WHODAS, 12-item World Health Organization Disability Assessment Schedule 2.0; WLQ, Work Limitations Questionnaire; WPAI, Work Productivity and Activity Impairment.
A strong and highly significant association was seen between the changes in PDQ-D-20 and WLQ productivity loss scores assessed by the partial correlation coefficient adjusted for age, sex, baseline PDQ-D-20, baseline WLQ productivity loss, disease duration, and disease severity at week 12 (primary study endpoint; r = 0.606; p < 0.001), and this association between PDQ-D-20 and WLQ productivity loss scores persisted at week 52 (r = 0.731; p < 0.001; Table 4). The correlations between changes in PDQ-D-20 scores and WLQ productivity loss were similar in first-treatment and switch patient groups at both timepoints.
TABLE 4. Analysis of partial correlation* between changes from baseline at weeks 12 and 52 in PDQ-D-20 score and WLQ productivity loss (full analysis set, observed cases)
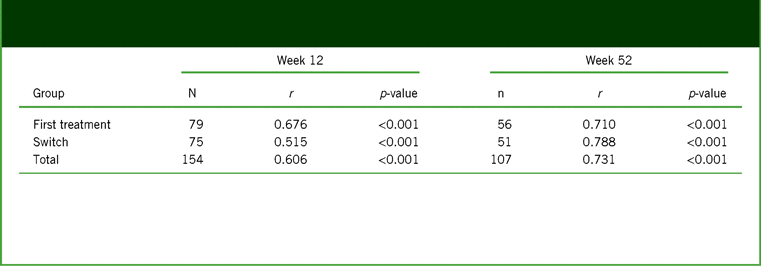
Notes: *Controlled for age, sex, baseline PDQ-D-20, baseline WLQ productivity loss, disease duration, and disease severity (baseline QIDS-SR, baseline CGI-S).
CGI-S, Clinical Global Impression–Severity; PDQ-D-20, 20-item Perceived Deficits Questionnaire–Depression; QIDS-SR, Quick Inventory of Depressive Symptomatology–Self-Report; WLQ, Work Limitations Questionnaire.
SEM analysis
Results of the SEM analyses are shown in Figure 5 and Supplementary Table 2. Patient-rated cognitive symptoms (PDQ-D-20 score), objective cognitive symptoms (DSST score), patient-rated depression severity (QIDS-SR score), and functional impairment (SDS total score or WLQ productivity loss score) were generally significantly dependent on the value of the previous assessment on the same scale at each timepoint over the 52 weeks of follow-up, ie, scores on each individual scale significantly predicted the subsequent score on the same scale (Supplementary Table 2).

FIGURE 5. One-lag structural equations models of standardized scores for (a) DSST, PDQ-D-20, SDS, and QIDS-SR; and (b) DSST, PDQ-D-20, WLQ productivity loss, and QIDS-SR. DSST, Digit Symbol Substitution Test; PDQ, 20-item Perceived Deficits Questionnaire–Depression; QIDS-SR, Quick Inventory of Depressive Symptomatology–Self-Report; SDS, Sheehan Disability Scale; WLQ, Work Limitations Questionnaire. Standardized regression coefficients (SRC) shown on individual paths; paths with an SRC having an absolute value of less than 0.2 are omitted.
Patient-rated cognitive symptoms (PDQ-D-20 score) at weeks 12 and 26 significantly predicted patient-rated functioning (SDS total score) at weeks 26 and 39, respectively (SRC, 0.22 and 0.27; both p <0.05). SRCs were adjusted for improvements in overall depressive symptoms, as the SEM included QIDS-SR scores. Patient-rated depression severity (QIDS-SR) scores did not significantly predict functioning outcomes (SDS or WLQ) at any subsequent timepoint; neither did PDQ-D-20 score predict WLQ productivity loss score at subsequent timepoints. Similarly, the objective cognitive performance measure (DSST) did not predict subjective measures of functioning (SDS or WLQ) at any subsequent timepoint.
PDQ-D-20 scores at weeks 12 and 39 significantly predicted QIDS-SR scores at the subsequent timepoints in the model including WLQ productivity loss (SRC, 0.20 and 0.27; p < 0.05 and p < 0.01, respectively). PDQ-D-20 score at week 26 also significantly predicted DSST score at week 39 in this model (SRC, –0.22; p < 0.05). In the model including SDS score, significant predictions were seen between PDQ-D-20 score at week 39 and QIDS-SR score at week 52 (SRC, 0.21), PDQ-D-20 score at week 26 and DSST score at week 39 (SRC, –0.22), and SDS score at week 26 and QIDS-SR score at week 39 (SRC, 0.22) (all p < 0.05). Both models (including either WLQ or SDS as functioning outcomes) fitted the data well with root mean square error of approximation and comparative fit index values being indicative of good fit (0.08 and 0.95, respectively, for the model including SDS total score; 0.08 and 0.94, respectively, for the model including WLQ productivity loss).
Safety and tolerability
Long-term treatment with vortioxetine was well tolerated. The most common treatment-emergent AEs were nausea (reported in 29.2% of treated patients), headache (11.9%), insomnia (9.1%), nasopharyngitis (6.8%), anxiety (6.4%), and dizziness (5.9%) (Supplementary Table 3). No new safety signals were observed. At 52 weeks, 99 patients (45.2%) had discontinued the study. The most common reason for study discontinuation was withdrawal of consent (42 patients, 19.2%). Only 16 patients (7.3%) discontinued the study due to an AE (9 in the first-treatment group and 7 in the switch group). Other reasons for discontinuation are shown in Supplementary Table 4.
Discussion
To our knowledge, this is the first study to examine the long-term effects of antidepressant therapy on cognition and functioning in working patients with MDD in a real-world setting. Patient demographic and disease characteristics at baseline were as expected for the general population presenting with MDD likely to be treated with antidepressants, supporting generalization of the study findings. Gainfully employed patients with MDD receiving vortioxetine in a real-life setting demonstrated clinically relevant improvements in mood, cognitive, and functional outcomes after continuous long-term treatment for up to 52 weeks. Work productivity improved over the 52 weeks of treatment by 8.9 percentage points on the WLQ productivity loss score, which ranges from 0 to 25% and assesses 4 dimensions of work productivity (time-management, physical demands, mental-interpersonal demands, and output demands).Reference Lerner, Amick, Rogers, Malspeis, Bungay and Cynn44
The highly significant association between improvements in cognitive symptoms (assessed using the PDQ-D-20) and workplace productivity loss previously reported after 12 weeks of vortioxetine treatment persisted at 52 weeks.Reference Chokka, Bougie, Rampakakis and Proulx40 These findings are in keeping with the results of a study in South Korea, in which MDD patients with greater severity of cognitive symptoms assessed by the PDQ-D-20 reported worse functional and work-related productivity outcomes, irrespective of depression severity.Reference Kim, Chalem, di Nicola, Hong, Won and Milea45 In a European study in patients with MDD who were either initiating or undergoing their first switch of antidepressant monotherapy (PERFORM), patient-reported cognitive symptoms (assessed using the shorter 5-item Perceived Deficit Questionnaire) were found to be independently associated with functional impairment, reduced work productivity, and lower quality of life throughout 2 years of follow-up.Reference Hammer-Helmich, Haro and Jönsson46, Reference Saragoussi, Christensen, Hammer-Helmich, Rive, Touya and Haro47
SEM analyses indicated that improvements in patient-rated cognitive symptoms preceded long-term improvements in functioning outcomes, and that cognitive symptoms at weeks 12 and 26 significantly predicted functioning at the subsequent visits, even when adjusting for improvement in depressive symptoms (QIDS-SR). In the European PERFORM study, similar SEM analyses showed patient-reported cognitive symptoms to be an important determinant of both subsequent functional impairment and depression severity throughout 2 years of follow-up in patients with MDD who were either initiating or undergoing their first switch of antidepressant monotherapy.Reference Larsen, Haro, Saragoussi and Hammer-Helmich43 The present study found subjectively rated cognitive symptoms (PDQ-D-20) to be a stronger predictor than objective cognitive performance (DSST) of subsequent functioning outcomes, suggesting that PDQ-D-20 and DSST provide distinct assessments of cognitive function. Other studies have also found discrepancies between subjective and objective measures of cognition in patients with MDD.Reference Lam, Iverson and Evans48–Reference Fava, Mahableshwarkar and Jacobson51 Collectively, such findings suggest that improvements in cognitive symptoms in patients with MDD may increase workplace productivity.
The significant improvements in both self-reported cognitive symptoms and cognitive performance assessed by the DSST seen in this study are consistent with results of previous studies of vortioxetine and reinforce these findings in a real-life setting.Reference McIntyre, Lophaven and Olsen33–Reference Baune, Sluth and Olsen38 The significant improvements in work productivity seen in the present study during treatment with vortioxetine are consistent with the findings of the Combined Medications to Enhance Depression Outcomes (CO-MED) trial, which demonstrated that work productivity outcomes improve significantly with antidepressant treatment and that early changes in work productivity are significant predictors of long-term clinical course.Reference Jha, Minhajuddin, Greer, Carmody, Rush and Trivedi52
In contrast to the results of the European PERFORM study,Reference Larsen, Haro, Saragoussi and Hammer-Helmich43 patient-rated depression severity (QIDS-SR) scores were not found to significantly predict functioning outcomes (SDS or WLQ) when simultaneously adjusting for improvements in cognitive symptom severity (PDQ-D-20 score) at any subsequent timepoint in the current SEM analyses. A reason for this result may be difference in sample sizes between the 2 studies. In the present study, unadjusted Pearson correlation coefficients did show significant associations between changes from baseline to week 52 for QIDS-SR scores and functioning outcomes (SDS and WLQ) as well as cognitive symptom severity (PDQ-D-20 score).
Depression and anxiety are frequently comorbid, with around 50% of patients with MDD also suffering from clinically significant levels of anxiety symptoms.Reference Fava, Rush and Alpert53 Anxiety has been shown to contribute to increased rates of suicide, poor response to treatment, and increased risk of chronicity and recurrence in patients with depression.Reference Seo, Jung and Kim54, Reference Gaspersz, Nawijn, Lamers and Penninx55 Significant improvement in the severity of anxiety symptoms was seen over the 52 weeks of vortioxetine treatment in the present study, from “severe” anxiety at baseline to “mild” at weeks 12 and 52. A highly significant correlation between anxiety symptoms and workplace productivity was also observed at weeks 12 and 52, highlighting a need for routine assessment and management of anxiety in patients with MDD for optimum outcomes.
High rates of treatment response and remission were seen after 52 weeks of vortioxetine treatment in this study. Overall, 77% of patients responded to treatment, and 56% of patients achieved remission after 52 weeks of vortioxetine. These rates are in keeping with those reported in a recent pooled analysis of data from 5 long-term, open-label extension studies of vortioxetine in patients with MDD.Reference Vieta, Loft and Florea32 In that analysis, response (defined as ≥50% improvement in Montgomery Åsberg Depression Rating Scale [MADRS] score) was achieved in 75% of patients at week 52 and remission (defined as MADRS total score ≤10) in 61%. The high rate of remission achieved in switch patients in the present study is particularly noteworthy, as these patients had a statistically significantly longer duration of MDD than those receiving vortioxetine as first treatment for the current depressive episode (11.0 versus 5.6 years, respectively). The remission rate of 67% seen in switch patients after 52 weeks of vortioxetine treatment in this study is high when considered in context of the second-line remission rates reported by the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) studyReference Rush, Trivedi and Wisniewski56; in both real-world studies, remission was defined as QIDS-SR total score ≤5. However, the high response and remission rates seen in patients switching to vortioxetine after inadequate response to a previous antidepressant in this real-world study are similar to those reported in previous randomized controlled studies of vortioxetine.Reference Thase, Mahableshwarkar, Dragheim, Loft and Vieta31, Reference Montgomery, Nielsen, Poulsen and Häggström57
Vortioxetine was well tolerated in this patient population. Safety and tolerability were consistent with previous reports of the short- and long-term tolerability of vortioxetine, Reference Baldwin, Chrones and Florea58 and with the data reported in the Canadian product monograph for vortioxetine (Trintellix).59 Common AEs included nausea, headache, and insomnia, and few patients discontinued the study due to AEs. The overall discontinuation rate of 45% is not unexpected for a long-term study, and few patients discontinued due to AEs (7.3%). This is similar to the overall discontinuation rate of 43% and a discontinuation rate due to AEs of 7.8% reported after 52 weeks of treatment with vortioxetine in a recent pooled analysis of data from 5 long-term, open-label extension studies.Reference Vieta, Loft and Florea32 Almost half of all patients who discontinued the AtWoRC study withdrew consent; as patients in this study were gainfully employed, it seems reasonable to assume that the need for regular study assessments over the 52 weeks of follow-up may have become burdensome.
A major strength of this study is that it was performed in a real-world setting with long-term follow-up. In addition, patient-reported outcome measures were used to assess disease severity and impact from the patient’s own perspective. Use of patient-reported outcomes is in keeping with the general move toward increased patient involvement in treatment decisions, and awareness of the limitations of clinical symptom-based measures in assessing recovery from mental illness in a way that is meaningful to patients.Reference Andresen, Caputi and Oades60–Reference Oliveira-Maia, Mendonça, Pessoa, Camacho and Gago63 Potential limitations include the open-label study design and the lack of a control group or active comparator; as such, the improvement observed was not controlled for any potential positive effect resulting from being included and assessed over time in the study. However, the AtWoRC study was undertaken primarily to assess the relationship between long-term changes in symptoms and workplace productivity in working patients with MDD treated with vortioxetine. The single-cohort study design was therefore appropriate, as the study was not designed to draw conclusions about the effectiveness of vortioxetine compared with other treatments.
Conclusions
In summary, results of the AtWoRC study demonstrate the long-term benefits of vortioxetine treatment in working patients with MDD in a real-world setting. Clinically relevant improvements in mood, anxiety and cognitive symptoms, work productivity, and functional outcomes were seen over the 52 weeks of treatment, as well as high rates of response and remission. A highly significant positive correlation was seen between changes in patient-reported cognitive symptoms and workplace productivity after 12 and 52 weeks of vortioxetine treatment, with results of SEM analyses confirming that improvements in patient-rated cognitive symptoms predicted long-term improvements in functional outcomes even when adjusting for improvement in depressive symptoms. These findings suggest that treating cognitive symptoms is clinically important in order to achieve functional recovery in patients with MDD.
Disclosures
Pratap Chokka has received honoraria from serving on advisory boards with Allergan, Lundbeck, Janssen, Pfizer, Sunovion, Purdue, and Shire. He has also had speaking engagements with Allergan, Lundbeck, Janssen, Pfizer, Sunovion, Shire, and Purdue. He has received research grants from Lundbeck and Janssen. Joanna Bougie is an employee of Lundbeck Canada. Jean Proulx was an employee of Lundbeck Canada at the time the study was conducted. Anders Holmegaard Tvistholm and Anders Ettrup are employees of H. Lundbeck A/S. The authors declare no conflicts of interest in this work.
Supplementary materials
To view supplementary material for this article, please visit https://doi.org/10.1017/S1092852919000786











