Introduction
For patients living with major depressive disorder (MDD), delays in treatment response can lead to nonadherence, multiple failed therapies, and negative health-related quality of life (HRQoL) outcomes.Reference IsHak, Mirocha and James 1 , Reference Rush, Trivedi and Wisniewski 2 Current guidelines recommend starting pharmacologic antidepressant therapy (ADT) or psychotherapy as an initial treatment option for patients with mild-to-moderate MDD and both pharmacotherapy and psychotherapy for those with moderate-to-severe MDD.Reference Gelenberg, Freeman and Markowitz 3 , 4 Standard-of-care (SOC) ADTs (eg, selective serotonin reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors) have several treatment-limiting side effects in some patients, including sexual dysfunction, weight gain, and disturbed sleep, which may contribute to nonadherence.Reference Wang, Han and Bahk 5 -Reference Samples and Mojtabai 8 Accordingly, guidelines recommend a “start low, go slow” approach to dosing ADTs when treating patients with MDD; since most SOC ADTs typically take several weeks to achieve maximal efficacy, patients are encouraged to continue these ADTs for 6 to 8 weeks before increasing the dose or switching to another agent.Reference Gelenberg, Freeman and Markowitz 3 , Reference Gautam, Jain, Gautam, Vahia and Grover 9 As many as ≥50% of patients may not respond (as measured by ≥50% reduction in 17-item Hamilton Rating Scale for Depression [HAMD-17] total score, a tool often used in clinical trials to assess depressive symptoms) to their initial ADT and will need changes to therapy—leading to a longer time to achieve therapeutic benefit, and in some instances, chronic treatment for sustained benefit.Reference Rush, Trivedi and Wisniewski 2 Patients in the STAR*D study who required >1 treatment step were less likely to achieve remission (HAMD-17 total score ≤7) and more likely to relapse than those who responded to their first ADT.Reference Rush, Trivedi and Wisniewski 2 A rapid response to treatment is associated with long-term remission, whereas patients with delayed response were more likely to experience residual symptoms, including anxiety, sleep disturbances, pain, and decreased concentration.Reference Romera, Pérez and Ciudad 6 , Reference Nierenberg, Husain and Trivedi 7 To prevent relapse, close attention should be placed on patients who require a relatively long time to achieve remission.Reference Kubo, Sakurai, Tani, Watanabe, Mimura and Uchida 10 Together, protracted treatment with SOC ADTs, residual symptoms, and delayed remission can lead to negative impacts on HRQoL, with ≥50% of patients facing severely impaired HRQoL after trying multiple lines of therapy.Reference IsHak, Mirocha and James 1 , Reference Kelly, Posternak and Alpert 11 In contrast, patients who experience a shorter time to response or remission can experience fewer mental, physical, and financial hardships compared with those who experience a longer time to response or remission.Reference Romera, Pérez and Ciudad 6 , Reference Arnaud, Suthoff, Tavares, Zhang and Ravindranath 12 The negative impacts of a relatively long time-to-effect with SOC ADTs underscore the need for novel therapies that can provide patients with MDD (including those experiencing suicidal ideation or behavior) improvements in depressive symptoms that are rapid (ie, within 1 week) and sustained. 13
Currently available interventions with rapid and sustained improvement
Electroconvulsive therapy (ECT), which involves applying electrical stimulation to a patient’s brain to produce a seizure, is an FDA-approved nonpharmacologic intervention associated with rapid and sustained effects in patients with severe treatment-resistant depression (TRD) and clinical emergencies that require rapid improvements in depressive symptoms (Table 1).Reference Kellner, Greenberg, Murrough, Bryson, Briggs and Pasculli 14 , Reference Li, Yao and Sun 15 ECT is believed to treat depression by inducing neurogenesis and increasing neurotrophic signaling and is considered an effective treatment option for patients with MDD. Transcranial magnetic stimulation (TMS) is another FDA-approved, rapid-acting nonpharmacologic therapy to treat adults with MDD for whom prior ADTs failed.Reference Cohen, Bikson, Badran and George 32 , Reference Croarkin and MacMaster 33 TMS noninvasively uses a strong pulsated magnetic field to stimulate a specific area of the cerebral cortex, leading to increased neural activity in the region.Reference Garnaat, Yuan, Wang, Philip and Carpenter 34 Although its precise mechanism of action is unclear, TMS is hypothesized to address inhibitory-excitatory imbalance between gamma-aminobutyric acid (GABA) and glutamate neurotransmission. Another form of TMS, the Stanford accelerated intelligent neuromodulation therapy, also received FDA approval to treat MDD in adults for whom prior ADTs failed.Reference Cole, Phillips and Bentzley 18 Both ECT and TMS approaches provide rapid improvements in depressive symptoms within 1 week that may be sustained for several weeks.Reference Husain, Rush and Fink 16 -Reference Cole, Phillips and Bentzley 18 , Reference Yesavage, Fairchild and Mi 35 However, a limitation of these approaches is that they require administration by trained providers at specialized treatment locations, in some cases over multiple sessions.
Table 1. Currently available and investigational treatments with rapid and sustained response in patients with MDD
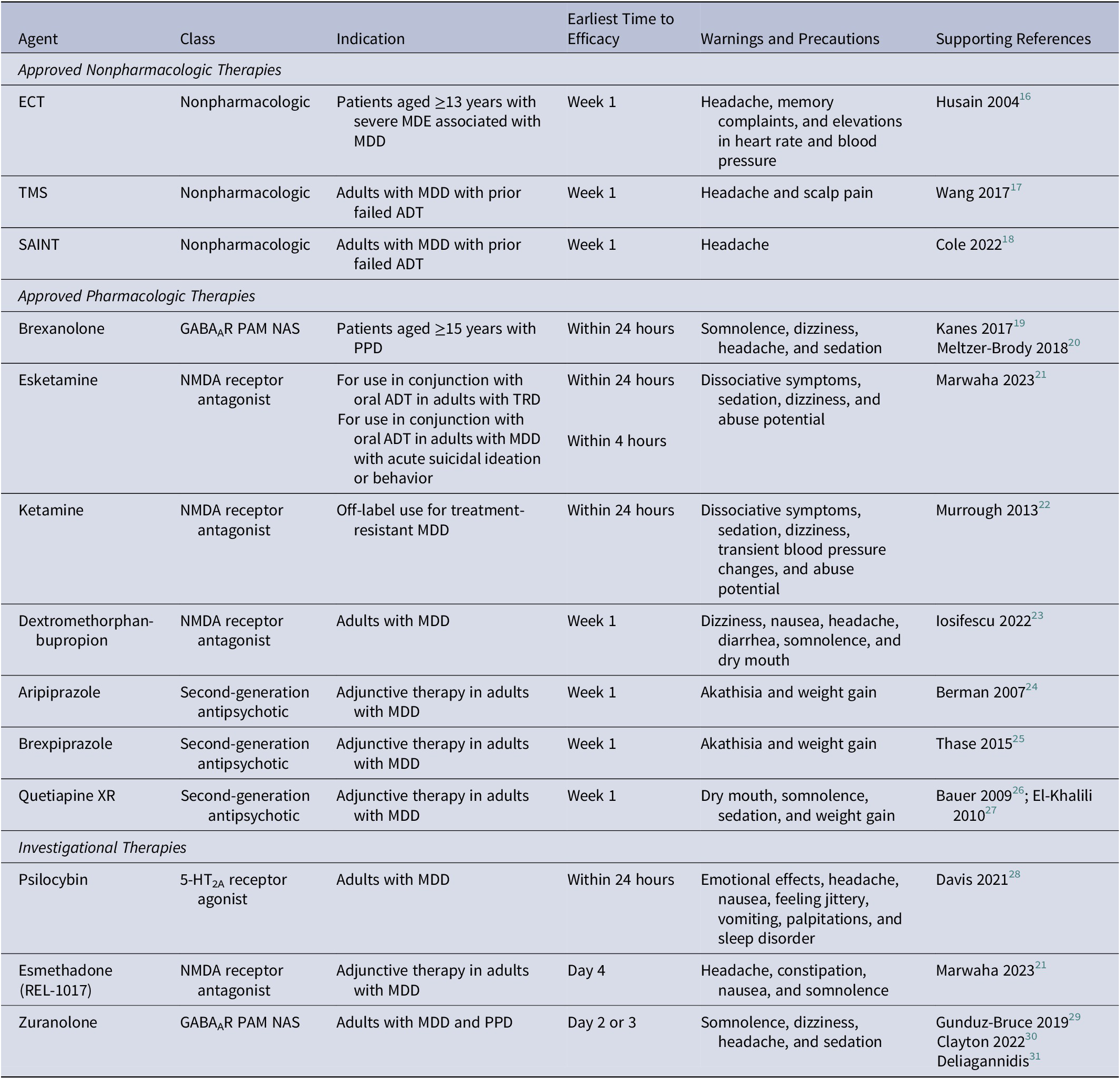
Abbreviations: 5-HT2A, 5-hydroxytryptamine type 2A; ADT, antidepressant therapy; ECT, electroconvulsive therapy; GABAAR, gamma-aminobutyric acid type A receptor; MDD, major depressive disorder; MDE, major depressive episode; NAS, neuroactive steroid; NMDA, N-methyl-D-aspartate; PAM, positive allosteric modulator; PPD, postpartum depression; SAINT, Stanford accelerated intelligent neuromodulation therapy; TMS, transcranial magnetic stimulation; TRD, treatment-resistant depression; XR, extended release.
Several pharmacotherapies with rapid and sustained effects are approved or used off-label as monotherapies or adjunct therapies for the treatment of MDD and other depressive disorders. Ketamine, esketamine, and dextromethorphan-bupropion are thought to act as N-methyl-D-aspartate (NMDA) receptor antagonists and elicit antidepressant effects through modulation of glutamate signaling (Table 1). These agents demonstrate rapid effects in as little as an hour, with improvements in depressive symptoms sustained up to 6 weeks.Reference Marwaha, Palmer, Suppes, Cons, Young and Upthegrove 21 -Reference Iosifescu, Jones and O’Gorman 23 However, ketamine and esketamine are limited by dissociative side effects and the potential for abuse, as well as the need for in-clinic visits to administer treatments.Reference Marwaha, Palmer, Suppes, Cons, Young and Upthegrove 21 Additionally, dextromethorphan is administered with bupropion, a potent cytochrome P450 2D6 inhibitor that increases the risk for drug-drug interactions and is associated with an increased risk of seizures. 36 The antidepressant activity of glutamatergic drugs supports the hypothesis that excitation-inhibition signaling in the brain is dysregulated in depression, suggesting that modulation of excitatory (glutamate) and inhibitory (GABA) signaling is a promising target for novel ADTs. Brexanolone, a neuroactive steroid and positive allosteric modulator (PAM) of GABAA receptors (GABAARs), is the only FDA-approved pharmacologic therapy for postpartum depression (PPD) and elicits rapid improvements in depressive symptoms in as early as 24 hours, but is limited by the need for a 60-hour intravenous infusion in a clinical setting.Reference Meltzer-Brody, Colquhoun and Riesenberg 20 , Reference Marwaha, Palmer, Suppes, Cons, Young and Upthegrove 21 , 37
Dopamine and serotonin partial agonist antipsychotics, aripiprazole and brexpiprazole, also provide rapid and sustained benefits, but their use is limited by the potential for psychomotor side effects such as akathisia.Reference Berman, Marcus and Swanink 24 , Reference Thase, Youakim and Skuban 25 Quetiapine is a norepinephrine reuptake inhibitor antipsychotic used as an adjunct to SOC ADTs that also demonstrates rapid and sustained benefits, but is associated with side effects (eg, weight gain) that may be treatment limiting.Reference Bauer, Pretorius, Constant, Earley, Szamosi and Brecher 26 , Reference El-Khalili, Joyce and Atkinson 27 While there are available therapies that, alone or in combination with SOC ADTs, may help to achieve rapid relief from depressive symptoms, their limitations, both practical and therapeutic, indicate a need for newer therapies.
Investigational pharmacologic interventions with the potential for rapid and sustained improvement
Psychedelics have emerged as a promising class of investigational therapies with the potential for rapid and sustained improvements in depressive symptoms for patients with MDD (Table 1). Psilocybin, a 5-hydroxy-tryptamine type 2A receptor agonist, is a psychedelic compound that can improve depressive symptoms in conjunction with psychotherapy, with studies demonstrating effects 1 week postdose that were sustained through 8 weeks without maintenance dosing.Reference Marwaha, Palmer, Suppes, Cons, Young and Upthegrove 21 , Reference Davis, Barrett and May 28 However, some patients treated with psilocybin have experienced emotional effects, including anxiousness, fear, and other emotional distress during treatment sessions, and use of psilocybin is limited by the need to receive treatment in a clinical setting and remain under supervision for several hours.Reference Marwaha, Palmer, Suppes, Cons, Young and Upthegrove 21 , Reference Davis, Barrett and May 28 , Reference Carhart-Harris, Giribaldi and Watts 38 Esmethadone, a novel NMDA receptor antagonist, can provide improvements in as little as 4 days of treatment in patients for whom up to 3 ADTs failed, but its long-term efficacy is unknown.Reference Marwaha, Palmer, Suppes, Cons, Young and Upthegrove 21
Modulation of GABAergic signaling is an evolving approach to treat MDD among investigational therapies (Table 1). Zuranolone is a GABAAR PAM and neuroactive steroid that is thought to upregulate GABAAR expression and enhance GABAergic signaling via synaptic and extrasynaptic GABAARs; zuranolone is currently in clinical development as an oral, once-daily, 14-day treatment course for MDD and PPD in adults.Reference Gunduz-Bruce, Silber and Kaul 29 , Reference Clayton, Deligiannidis and Lasster 30 , Reference Althaus, Ackley and Belfort 39 -Reference Hoffmann, Nomikos and Kaul 41 Targeting both synaptic and extrasynaptic GABAARs distinguishes zuranolone from other GABAergic drugs, such as benzodiazepines, which only target synaptic receptors.Reference Althaus, Ackley and Belfort 39 Across several clinical trials, patients with MDD or PPD treated with zuranolone experienced rapid improvements in depressive symptoms that were sustained for several weeks beyond the 14-day treatment course; the most common side effects included headache, dizziness, somnolence, and sedation, which were mostly mild or moderate in severity.Reference Gunduz-Bruce, Silber and Kaul 29 , Reference Deligiannidis, Meltzer-Brody and Gunduz-Bruce 31 , Reference Clayton, Lasser and Brown 42 Interim results from an ongoing, open-label, longitudinal study of zuranolone showed that most patients who responded at day 15 received ≤2 treatment courses during ≤1 year of follow-up, suggesting that response to treatment is rapid and sustained.Reference Cutler, Aaronson and Mattingly 43 , Reference Cutler, Aaronson and Mattingly 44
Conclusions
The therapeutic landscape for MDD is shifting to better address the delay in relief of symptoms experienced with current SOC ADTs. Patients with MDD with delayed response to treatment often experience worse clinical outcomes and lower HRQoL compared with those with rapid response. Some of the available treatments for patients with MDD demonstrate rapid improvement in depressive symptoms (eg, ECT, TMS, and esketamine) but are targeted for those with severe depression or TRD and require treatment at a specialized site with direct supervision by healthcare professionals, which may be particularly challenging for patients who are unable to find time to visit a treatment center or for patients in rural areas. Novel investigational therapeutic options offer the potential to achieve therapeutic efficacy within 1 week of treatment and demonstrate positive benefits, including a shorter time to remission, fewer residual symptoms, and greater HRQoL. Furthermore, several of the investigational agents are not associated with some of the treatment-limiting side effects commonly observed with SOC ADTs, such as sexual dysfunction, weight gain, and disturbed sleep. For some patients, these investigational therapies demonstrate favorable benefit-to-risk profiles relative to SOC ADTs. As more pharmacologic options with rapid and sustained effects enter clinical development and become available, it will be necessary to assess the accessibility of these treatment options and to adjust treatment expectations of patients and physicians. As such, a re-evaluation of the current “start low, go slow” clinical approach to pharmacotherapy for depression is critical, and will likely shift to one that targets individualized therapy that rapidly improves depressive symptoms without a significant side effect burden and need for chronic treatment.
Acknowledgements
The author thanks Matthew Brown, PhD, and Jay Parekh, PharmD, of Symbiotix, LLC, and Ryan Coleman, PhD, of AlphaBioCom, LLC, for providing medical writing assistance and editorial support, which were funded by Sage Therapeutics, Inc., and Biogen Inc.
Financial support
This work was funded by Sage Therapeutics, Inc., and Biogen Inc. During the development process, Sage Therapeutics, Inc., and Biogen Inc., had the opportunity to review and comment on the manuscript, and the author retained full editorial control and provided final approval on all content. Medical writing assistance and editorial support were provided by Symbiotix, LLC, and AlphaBioCom, LLC, and funded by Sage Therapeutics, Inc., and Biogen Inc. The author has received research funding from Sage Therapeutics, Inc., AbbVie, Acadia, Alector, Athira, Avanir, Eisai, Lilly, LivaNova, and Teva.
Author contribution
Conceptualization: G.A.; Writing—original draft: G.A.; Writing—review and editing: G.A.
Disclosure
The author has served as a consultant for Sage Therapeutics, Inc., Biogen Inc., AbbVie, Acadia, Alfa Sigma, Alkermes, Avanir, Axsome, Janssen, Lundbeck, Myriad, Otsuka, Sunovion, Takeda, Teva, and Vanda; and served as a speaker for Sage Therapeutics, Inc., Biogen Inc., AbbVie, Acadia, Alfa Sigma, Alkermes, Avanir, Axsome, Intracellular, Janssen, Lundbeck, LivaNova, Myriad, Neurocrine, Otsuka, Sunovion, Takeda, and Teva.



