Introduction
Long-acting injectable (LAI) antipsychotics provide continuous plasma antipsychotic exposure over the extended period between injectionsReference Ceskova and Silhan 1 and may reduce the risk of undetected medication gaps during the course of treatment.Reference Brissos, Veguilla, Taylor and Balanza-Martinez 2 , Reference Citrome 3 Nonetheless, approximately 30% or fewer outpatients with schizophrenia are prescribed LAIs, even among those considered poorly adherent.Reference Barnes, Shingleton-Smith and Paton 4 Statement 10 of the 2020 American Psychiatric Association (APA) Practice Guideline for Treatment of Patients with Schizophrenia addresses the role of LAIs and reads “APA suggests … that patients receive treatment with a LAI antipsychotic medication if they prefer such treatment or if they have a history of poor or uncertain adherence.” 5 For patients newly initiated on LAIs and their clinicians, prior experience with antipsychotic medications is most often with oral formulations.Reference Barnes, Shingleton-Smith and Paton 4 , Reference Munday, Greene, Chang, Hartry, Yan and Broder 6 Therefore, a possible barrier to the APA recommendation is a lack of familiarity with some of the technical aspects of using LAIs. While it is beyond the scope of this paper to cover all barriers to the use of LAIs, they may include hesitation as to how to bring up the topic of LAIs using person-centered orientation, how to initiate or switch to an LAI, or how to choose a specific LAI regimen in a way that is consistent with the clinician’s approach to prescribing the oral antipsychotic counterpart.Reference Weiden, Roma, Velligan, Alphs, DiChiara and Davidson 7 – Reference Sajatovic, Ross and Legacy 12
This paper focuses on the LAI aripiprazole lauroxil (AL; Aristada®, Alkermes, Inc, Waltham, MA) and considers one aspect of the broader implementation challenge, the selection of an LAI dosing regimen. AL is a prodrug of aripiprazole that is formulated as a suspension of micrometer-sized drug particles of AL that are slowly released into the bloodstream and converted to aripiprazole, allowing for dose intervals of up to 2 months depending on the dose administered.Reference Farwick, Hickey, Jacobs, Faldu, Vandiver and Weiden 13 , Reference Jain, Meyer, Wehr, Rege, Von Moltke and Weiden 14 When prescribing an LAI antipsychotic for an individual patient, clinicians consider patient characteristics such as course of illness, past history of response to and tolerance of other antipsychotics, likelihood of adherence, and treatment preferences.Reference Meyer 11 , Reference Sajatovic, Ross and Legacy 12 , Reference Geerts, Martinez and Schreiner 15 , Reference Sajatovic, Ross and Legacy 16 After selecting an LAI, the clinician must choose from among available dosing regimens, which vary according to dosage strength (ie, milligram of drug per injection given) and injection interval (recommended time between injections).
Following each administration of an LAI, the drug is slowly released into the plasma over a time period governed by its pharmacokinetics. For AL, this includes slow dissolution after injection, enzymatic cleavage by esterases followed by hydrolysis to yield active aripiprazole, and elimination via cytochrome P450 enzymes in the liver.Reference Hard, Mills, Sadler, Turncliff and Citrome 17 Clinicians or patients unfamiliar with the use of LAIs might misconstrue the regimen with greater milligram per injection as necessarily representing a “higher dose” producing greater plasma concentrations. However, the average steady-state plasma drug concentrations at a given time point are a function of both the dosage strength and the injection interval: after reaching steady state (ie, the point of dynamic equilibrium when there is no substantial difference in the average plasma drug concentrations from one injection to the next), a regimen with a higher dosage strength over a longer injection interval may produce plasma drug concentrations roughly comparable to those of a regimen with a lower dosage strength over a shorter injection interval. Multiple combinations of LAI injection dosage strengths and injection intervals can be tested or modeled during the drug development process, providing clinicians a range of possible dosing regimen options.
AL is an atypical antipsychotic indicated for the treatment of schizophrenia in adults. 18 It has five dosing regimens using four dosage strengths and three approved injection intervals: AL 441 mg, 662 mg, and 882 mg can be given monthly; AL 882 mg can also be given every 6 weeks; and AL 1064 mg is approved for every 2 months. The safety and efficacy of AL was established using 441 mg monthly and 882 mg monthly regimens in a phase 3 clinical trial in patients experiencing an acute exacerbation or relapse of schizophrenia.Reference Meltzer, Risinger and Nasrallah 19 In this pivotal study, both AL 441 mg monthly and 882 mg monthly regimens demonstrated significant efficacy vs placebo and no clinically relevant dose-related differences in efficacy or safety were observed between AL doses.Reference Meltzer, Risinger and Nasrallah 19 The three additional regimens were approved based on U.S. Food and Drug Administration guidance that allows for the use of pharmacokinetic bridging studies and population pharmacokinetic models; details have been reviewed elsewhere.Reference Hard, Mills, Sadler, Turncliff and Citrome 17 , 18 , Reference Hard, Mills, Sadler, Wehr, Weiden and von Moltke 20 , Reference Hard, Wehr, Sadler, Mills and von Moltke 21 Previous articles have reviewed initiation regimens for ALReference Jain, Meyer, Wehr, Rege, Von Moltke and Weiden 14 and switching from oral antipsychotics.Reference Weiden, Du, Liu and Stanford 22 This article illustrates the relationships between the five AL regimens to increase clinicians’ understanding of their prescribing options for AL and reduce the potential for confusion between the multiple alternatives.
Methods
In this article, plasma aripiprazole concentrations are simulated to illustrate the relationship between different AL dosing regimens and the expected plasma aripiprazole concentrations produced over time. The previously published population pharmacokinetic modelReference Hard, Wehr, Sadler, Mills and von Moltke 21 was used to generate 500 individual simulated concentration–time profiles for each AL regimen. The median concentrations calculated for each regimen are shown from AL initiation through week 56 in Figure 1A. The selection of an AL regimen is based on plasma aripiprazole concentrations at steady state; therefore, to understand the characteristics that differentiate the available AL dosing regimens, it is important to examine the plasma aripiprazole concentrations at steady state, not considering the early concentrations during the LAI initiation period. For illustrative purposes, the steady-state plasma concentration profiles starting 6 months after AL regimen initiation (see JainReference Jain, Meyer, Wehr, Rege, Von Moltke and Weiden14 for details) are shown in Figure 1B. Figure 1 also illustrates the relationship between steady-state plasma concentrations over time (Figure 1B) and the range of plasma concentrations averaged over injection interval at steady state (C avg,ss), shown as box plots in Figure 1C. C avg,ss was calculated as the area under the concentration over time curve for one injection interval, divided by the interval duration. The boxes in Figure 1C represent the 25th to 75th percentiles of C avg,ss starting at 48 weeks.
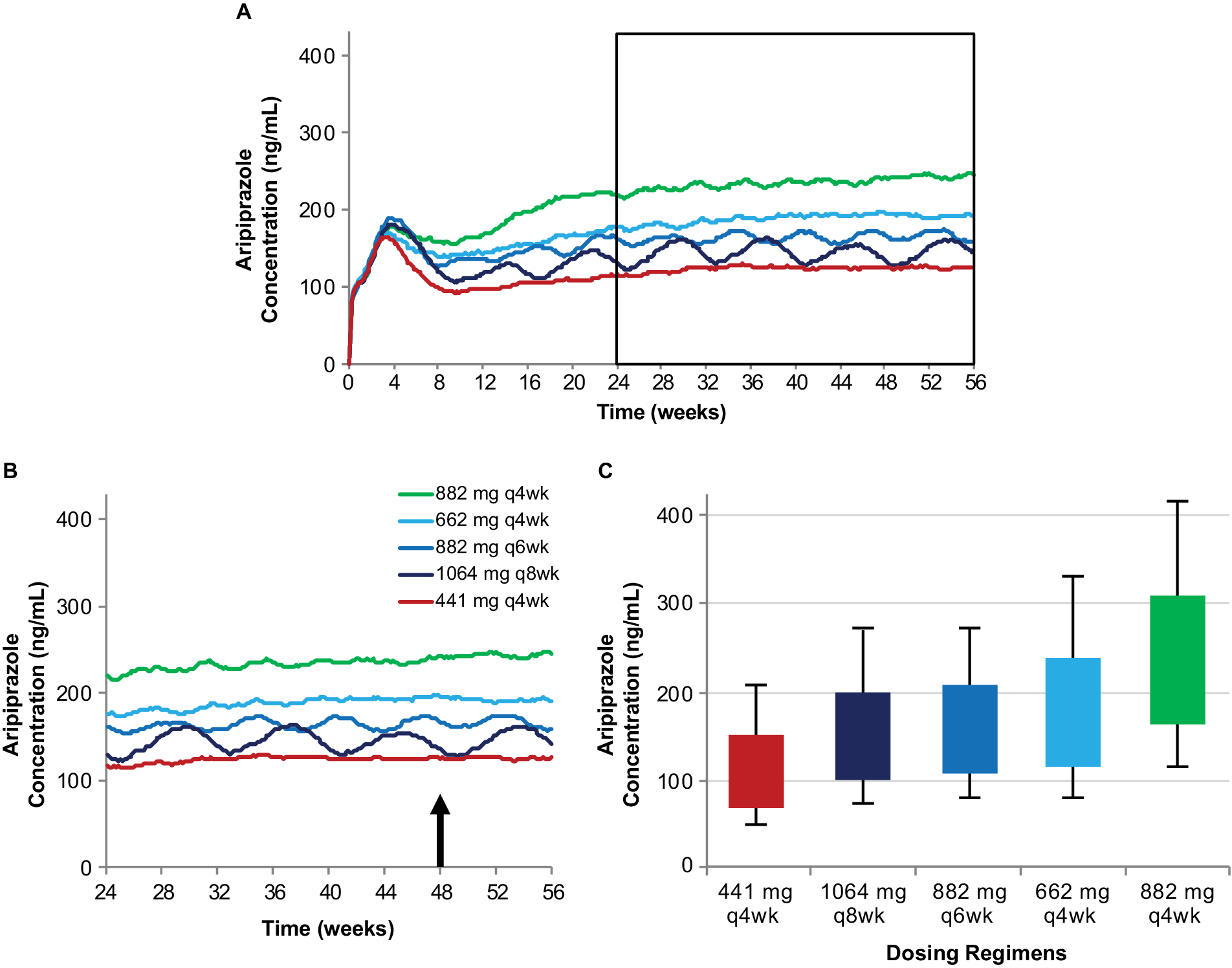
Figure 1. Simulated plasma aripiprazole concentrations associated with each of the AL dosing regimens (A) and the relationship between steady-state plasma aripiprazole concentrations over time (B) and average steady-state concentration over an injection interval (C). Model-basedReference Hard, Wehr, Sadler, Mills and von Moltke 21 simulations of median plasma aripiprazole concentrations associated with the five AL regimens, each initiated using a one-day regimen of ALNCD + a single 30 mg oral aripiprazole tablet. Steady-state concentrations from the timeframe shown boxed in panel A are expanded in panel B. Box plots in panel C represent the range of concentrations observed in simulations for the same five regimens (started using a 21-day oral aripiprazole initiation regimen) based on average steady-state concentration for the injection interval starting at week 48 (arrow in panel B). Boxes, 25th to 75th percentiles; whiskers, 10th and 90th percentiles; adapted from Hard et alReference Hard, Mills, Sadler, Wehr, Weiden and von Moltke 20 and used with permission. Abbreviations: AL, aripiprazole lauroxil; ALNCD, AL NanoCrystal® Dispersion; q4wk, every 4 weeks; q6wk, every 6 weeks; q8wk, every 8 weeks.
Results and Discussion
AL dosing regimens
The efficacy of AL for the treatment of schizophrenia was established in the 12-week pivotal study of the 441 mg and 882 mg monthly regimens.Reference Meltzer, Risinger and Nasrallah 19 Steady-state plasma aripiprazole concentrations achieved with these two monthly regimens, characterized using population pharmacokinetic modeling in Figure 2A, represent the upper and lower bounds of the range of concentrations associated with the known efficacy of AL therapy. Average concentrations for these two regimens at steady state are shown in box plots in Figure 2B. It is important to note that these are median concentrations and thus mark the midpoints for each regimen; half the simulations for each regimen resulted in concentrations below the respective median, and half above.
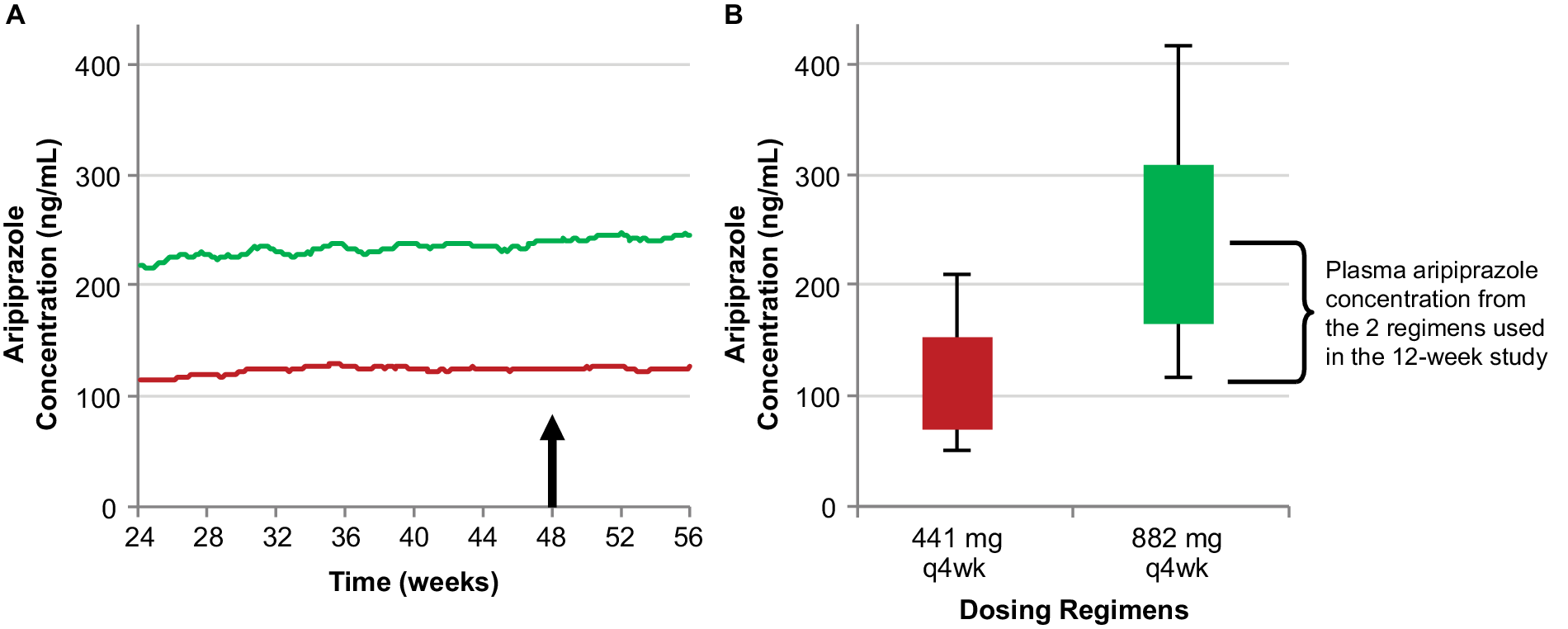
Figure 2. The relationship between AL 441 mg and 882 mg monthly regimens used in the 12-week pivotal study (Meltzer et alReference Meltzer, Risinger and Nasrallah 19) and their resulting steady-state plasma aripiprazole concentrations. Median simulated steady-state plasma aripiprazole concentrations over time (A); average simulated steady-state aripiprazole concentrations for the same regimens started using 21-day oral aripiprazole (B), calculated for the injection interval starting at week 48 (arrow in panel A). Boxes, 25th to 75th percentiles; whiskers, 10th and 90th percentiles; adapted from Hard et alReference Hard, Mills, Sadler, Wehr, Weiden and von Moltke 20 and used with permission. Abbreviation: AL, aripiprazole lauroxil; q4wk, every 4 weeks.
After the efficacy of the 441 mg and 882 mg monthly AL regimens was established in the pivotal study,Reference Meltzer, Risinger and Nasrallah 19 three additional AL regimens were developed through subsequent pharmacokinetic studies and pharmacokinetic modeling in which the steady-state plasma aripiprazole concentrations produced by the dosing regimens in the AL pivotal study served as lower (441 mg monthly) and upper (882 mg monthly) boundaries. In simulations, median estimated steady-state plasma aripiprazole concentrations after administration of AL 662 mg monthly, 882 mg every-6-weeks, and 1064 mg every-2-months regimens fall between the steady-state plasma concentrations for the AL 441 mg and 882 mg monthly regimens (Figure 1). Consequently, all five labeled AL regimens provide plasma aripiprazole concentrations within the range associated with established AL efficacy.
Considerations when selecting an AL regimen
Plasma drug concentration and dosage strength
The 441 mg and 882 mg monthly regimens deliver the lower and upper bounds, respectively, of plasma aripiprazole concentrations among the available AL regimens, while the 662 mg dosage strength with a monthly injection interval results in plasma aripiprazole concentrations between these two regimens, providing a third, intermediate AL regimen using the monthly injection interval (Figure 3).Reference Hard, Mills, Sadler, Turncliff and Citrome17
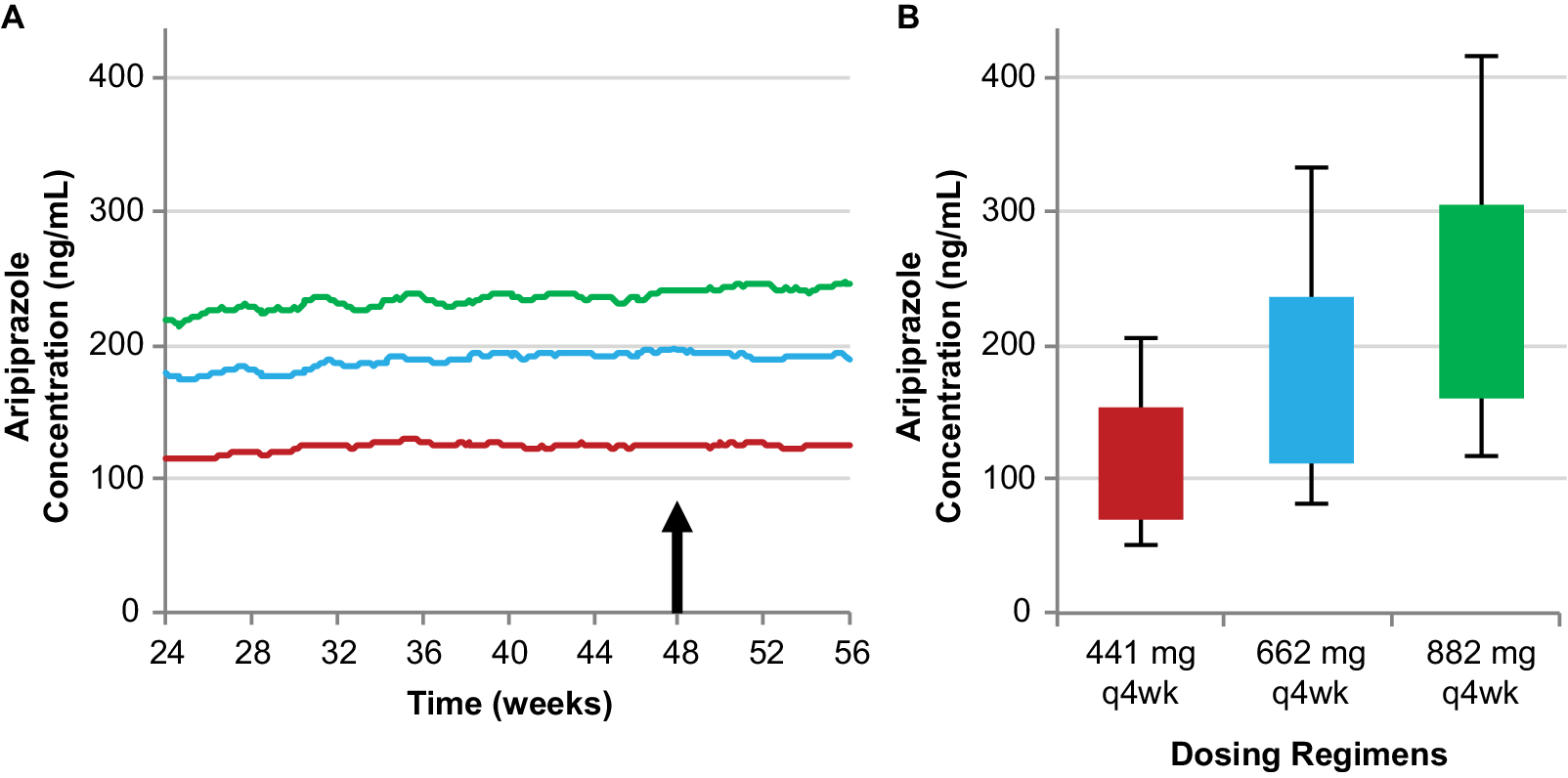
Figure 3. Simulated steady-state plasma aripiprazole concentrations produced by the 662 mg monthly regimen are intermediate between the 441 mg and 882 mg monthly regimens. Median simulated steady-state plasma aripiprazole concentrations over time (A); average simulated steady-state aripiprazole concentrations for the same regimens started using 21-day oral aripiprazole (B), calculated for the injection interval starting at week 48 (arrow in panel A). Boxes, 25th to 75th percentiles; whiskers, 10th and 90th percentiles; adapted from Hard et alReference Hard, Mills, Sadler, Wehr, Weiden and von Moltke 20 and used with permission. Abbreviation: AL, aripiprazole lauroxil; q4wk, every 4 weeks.
Thus, clinicians can pick a dosing regimen that provides plasma aripiprazole concentrations associated with the lowest or highest AL plasma concentrations with established efficacy for the monthly injection interval or choose an intermediate dosing regimen.
Injection interval
The monthly injection interval yields AL dose regimens that provide lower, intermediate, and upper plasma concentrations based on the 441 mg monthly, 662 mg monthly, and 882 mg monthly regimens, respectively. However, as Figure 4 illustrates, three AL injection intervals (monthly, every 6 weeks, and every 2 months) provide steady-state plasma aripiprazole concentrations that are intermediate between the upper and lower plasma concentrations produced by the 441 mg monthly and 882 mg monthly regimens, as demonstrated in the AL population pharmacokinetics modeling studies.Reference Hard, Mills, Sadler, Turncliff and Citrome17, Reference Hard, Mills, Sadler, Wehr, Weiden and von Moltke20 Each injection interval has a corresponding approved dosage strength that provides intermediate plasma aripiprazole concentrations: 662 mg monthly, 882 mg every 6 weeks, and 1064 mg every 2 months; Figure 5 compares the injection schedules for these intermediate options. Because a lower AL dosage strength given at shorter intervals and a higher dosage strength given at longer intervals can provide similar average plasma aripiprazole concentrations, the three intermediate dosage strength/injection interval combinations are associated with comparable plasma aripiprazole concentrations over time, as indicated by the box plots in Figure 4.
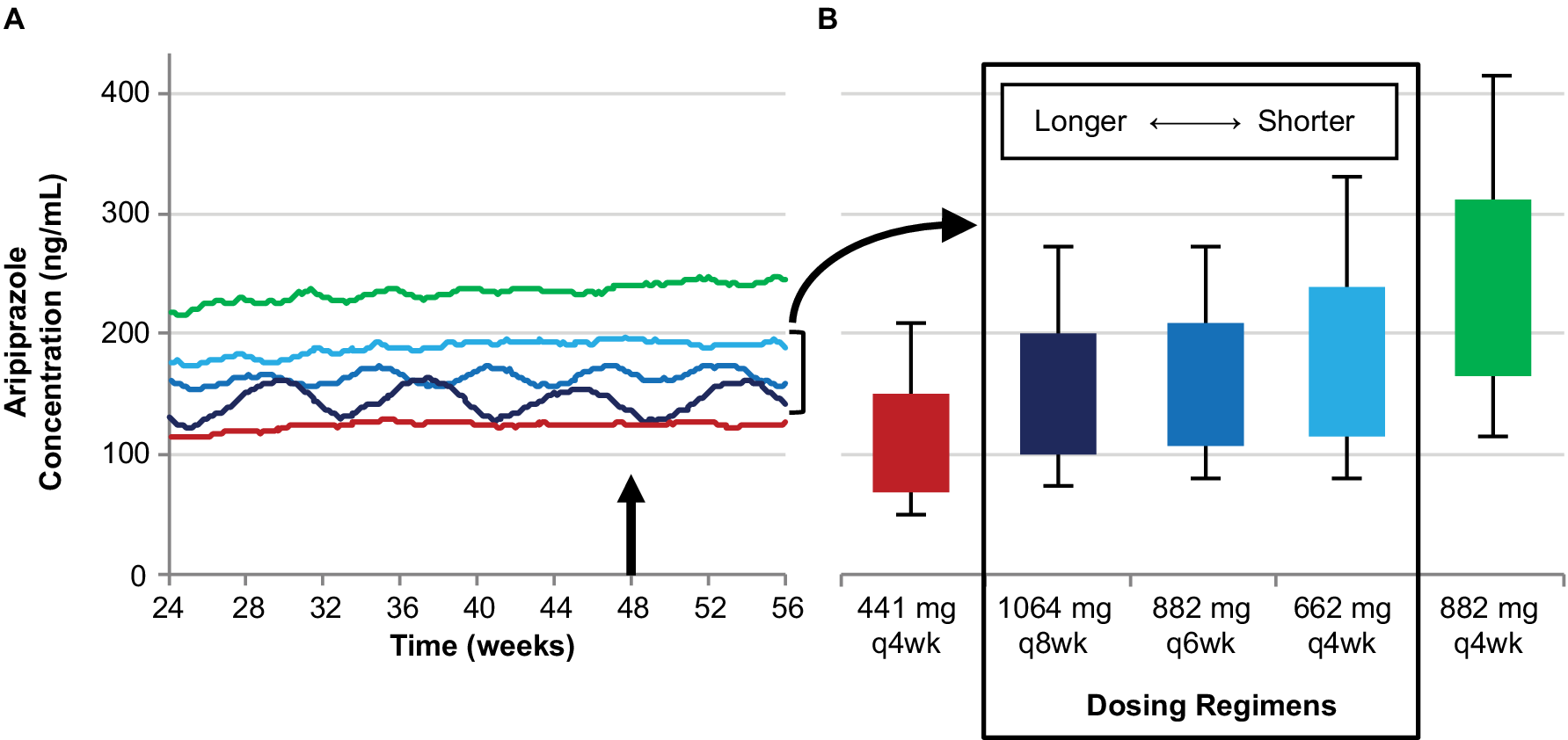
Figure 4. Three AL regimens provide intermediate plasma aripiprazole concentrations using different injection intervals.
Simulated steady-state plasma aripiprazole concentrations based on the 662 mg monthly, 882 mg every-6-week, and 1064 mg every-2-month regimens fall between those for the 441 mg and 884 mg monthly regimens. Median simulated steady-state plasma aripiprazole concentrations over time (A); average simulated steady-state aripiprazole concentrations for the same regimens started using 21-day oral aripiprazole (B), calculated for the injection interval starting at week 48 (arrow in panel A). Boxes, 25th to 75th percentiles; whiskers, 10th and 90th percentiles; adapted from Hard et alReference Hard, Mills, Sadler, Wehr, Weiden and von Moltke 20 and used with permission. AL, aripiprazole lauroxil; q4wk, every 4 weeks; q6wk, every 6 weeks; q8wk, every 8 weeks.
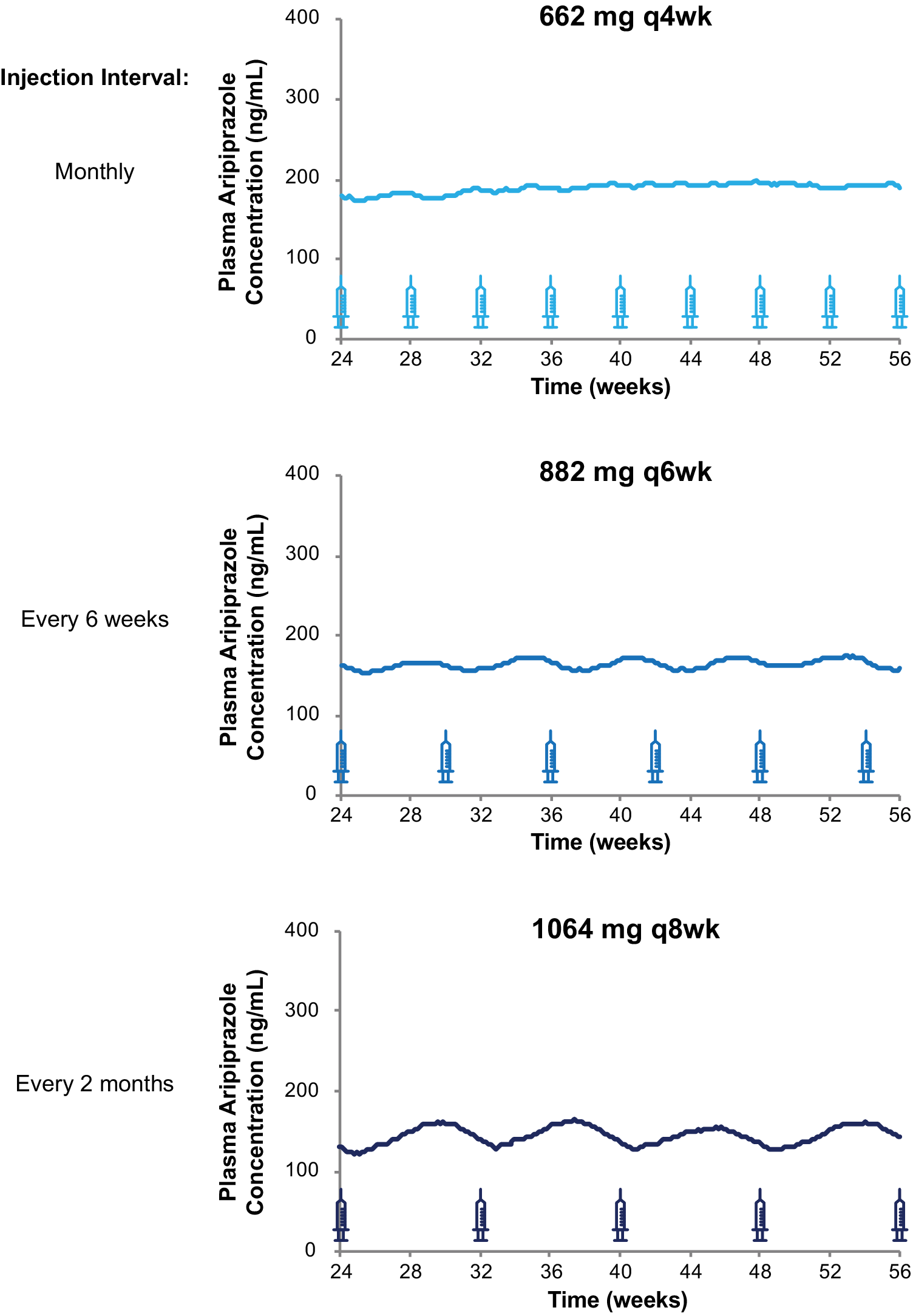
Figure 5. Injection schedules and plasma aripiprazole concentrations for three intermediate AL dosing regimens. Model-basedReference Hard, Wehr, Sadler, Mills and von Moltke 21 simulations of median plasma aripiprazole concentrations at steady state associated with the 662 mg monthly, 882 mg every-6-week, and 1064 mg every-2-month AL regimens. Syringes indicate injection days. Abbreviation: AL, aripiprazole lauroxil; q4wk, every 4 weeks; q6wk, every 6 weeks; q8wk, every 8 weeks.
Limitations
It is important to note that the terminology describing monthly or every-2-months injection intervals (used in the AL label) and every-4-weeks or every-8-weeks injection intervals (used in AL clinical trial study designs) can vary depending on context, but these injection intervals are generally considered equivalent, covering similar time periods in clinical use.Reference Lertxundi, Hernandez and Medrano 23 A small degree of variability in AL injection timing (ie, injection intervals of monthly vs every 4 weeks or every 2 months vs every 8 weeks) would not have a clinically significant impact on plasma aripiprazole concentrations over the injection interval.Reference Hard, Mills, Sadler, Turncliff and Citrome 17 The AL dosing regimens are characterized in this article as “upper,” “lower,” or “intermediate” regimens based on the expected steady-state plasma aripiprazole concentrations associated with each; no efficacy differences are expected between the regimens.
Conclusions
This paper illustrates the importance of integrating dosage strength and injection interval when considering LAI dosing regimens. Many clinicians have greater experience with oral antipsychotics and may be less familiar with LAI pharmacokinetics as they relate to different dosing regimens. However, an understanding of the relationship between dosage strength, injection interval, and expected plasma drug levels can help clarify distinctions between dosing regimens. In the case of AL, pharmacokinetic modeling of steady-state concentrations shows that regimens using the three injection intervals, in combination with specific dosage strengths, all provide adequate plasma concentrations of aripiprazole for the full course of the injection interval.Reference Hard, Mills, Sadler, Turncliff and Citrome 17 , 18 , Reference Hard, Mills, Sadler, Wehr, Weiden and von Moltke 20 , Reference Hard, Wehr, Sadler, Mills and von Moltke 21 When prescribing an AL regimen, clinicians can weigh this information, together with patient factors, to help make better choices for their patients’ clinical circumstances.
Funding
This study was sponsored by Alkermes, Inc. Medical writing and editorial support were provided by Kathleen M. Dorries, PhD, of Peloton Advantage, LLC (Parsippany, NJ), an OPEN Health company, and funded by Alkermes, Inc. (Waltham, MA). The authors are entirely responsible for the scientific content of this article.
Disclosures
Roger W. Sommi is a consultant to, and has received an honorarium and research funding from, Alkermes, Inc. Angela Wehr, Sejal Faldu, and Peter J. Weiden are former employees of Alkermes, Inc., and may own stock/options in the company. Bhaskar Rege and Yangchun Du are employees of Alkermes, Inc., and may own stock/options in the company.







