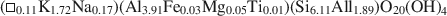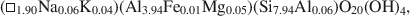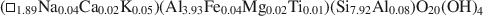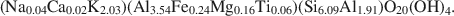Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Anovitz, Lawrence M.
Perkins, Dexter
and
Essene, Eric J.
1991.
Metastability in Near-Surface Rocks of Minerals in The System Al2O3-SiO2-H2O.
Clays and Clay Minerals,
Vol. 39,
Issue. 3,
p.
225.
Aja, Stephen U
1991.
Illite equilibria in solutions: III. A re-interpretation of the data of Sass et al. (1987).
Geochimica et Cosmochimica Acta,
Vol. 55,
Issue. 11,
p.
3431.
Aja, Stephen U
Rosenberg, Philip E
and
Kittrick, James A
1991.
Illite equilibria in solutions: I. Phase relationships in the system K2OAl2O3SiO2H2O between 25 and 250°C.
Geochimica et Cosmochimica Acta,
Vol. 55,
Issue. 5,
p.
1353.
Ohr, Matthias
Halliday, Alex N.
and
Peacor, Donald R.
1991.
Sr and Nd isotopic evidence for punctuated clay diagenesis, Texas Gulf Coast.
Earth and Planetary Science Letters,
Vol. 105,
Issue. 1-3,
p.
110.
Aja, Stephen U.
and
Rosenberg, Philip E.
1992.
The Thermodynamic Status of Compositionally-Variable Clay Minerals: A Discussion.
Clays and Clay Minerals,
Vol. 40,
Issue. 3,
p.
292.
Jasmund, K.
1993.
Tonminerale und Tone.
p.
168.
Jiang, Wei-Teh
and
Peacor, Donald R.
1994.
Prograde Transitions of Corrensite and Chlorite in Low-Grade Pelitic Rocks from the Gaspé Peninsula, Quebec.
Clays and Clay Minerals,
Vol. 42,
Issue. 5,
p.
497.
Li, Gejing
Peacor, Donald R.
Merriman, R. J.
and
Roberts, B.
1994.
The Diagenetic to Low-Grade Metamorphic Evolution of Matrix White Micas in the System Muscovite-Paragonite in a Mudrock from Central Wales, United Kingdom.
Clays and Clay Minerals,
Vol. 42,
Issue. 4,
p.
369.
Jiang, Wei-Teh
Peacor, Donald R.
and
Essene, Eric J.
1994.
Clay Minerals in the MacAdams Sandstone, California: Implications for Substitution of H3O+ and H2O and Metastability of Illite.
Clays and Clay Minerals,
Vol. 42,
Issue. 1,
p.
35.
Essene, E. J.
and
Peacor, D. R.
1995.
Clay Mineral Thermometry—A Critical Perspective.
Clays and Clay Minerals,
Vol. 43,
Issue. 5,
p.
540.
Komninou, A.
and
Sverjensky, D. A.
1995.
Pre-ore hydrothermal alteration in an unconformity-type uranium deposit.
Contributions to Mineralogy and Petrology,
Vol. 121,
Issue. 1,
p.
99.
Leikine, M.
Medina, F.
and
Ahmamou, M.
1996.
Lack of low-grade metamorphism in the Triassic formations of the Argana basin, Morocco: an illite crystallinity re-evaluation.
Journal of African Earth Sciences,
Vol. 22,
Issue. 4,
p.
565.
Nieto, F.
Ortega-Huertas, M.
Peacor, D. R.
and
Arostegui, J.
1996.
Evolution of Illite/Smectite from Early Diagenesis Through Incipient Metamorphism in Sediments of the Basque-Cantabrian Basin.
Clays and Clay Minerals,
Vol. 44,
Issue. 3,
p.
304.
Yates, Douglas M.
and
Rosenberg, Philip E.
1997.
Formation and stability of endmember illite: II. Solid equilibration experiments at 100 to 250°C and Pv,soln.
Geochimica et Cosmochimica Acta,
Vol. 61,
Issue. 15,
p.
3135.
Li, Gejing
Peacor, Donald R.
and
Coombs, Douglas S.
1997.
Transformation of Smectite to Illite In Bentonite and Associated Sediments from Kaka Point, New Zealand: Contrast in Rate and Mechanism.
Clays and Clay Minerals,
Vol. 45,
Issue. 1,
p.
54.
Aja, Stephen U.
1997.
Comment on “Formation and stability of endmember illite: I. Solution equilibration experiments at 100 to 250°C and Pv,soln” by D. M. Yates and P. E. Rosenberg.
Geochimica et Cosmochimica Acta,
Vol. 61,
Issue. 20,
p.
4455.
LIVI, K. J. T.
VEBLEN, D. R.
FERRY, J. M.
and
FREY, M.
1997.
Evolution of 2:1 layered silicates in low‐grade metamorphosed Liassic shales of Central Switzerland.
Journal of Metamorphic Geology,
Vol. 15,
Issue. 3,
p.
323.
Essene, E. J.
and
Peacor, D. R.
1997.
Illite and Smectite: Metastable, Stable or Unstable? Further Discussion and a Correction.
Clays and Clay Minerals,
Vol. 45,
Issue. 1,
p.
116.
Hover, Victoria C.
Walter, Lynn M.
Peacor, Donald R.
and
Martini, Anna M.
1999.
Mg-Smectite Authigenesis in a Marine Evaporative Environment, Salina Ometepec, Baja California.
Clays and Clay Minerals,
Vol. 47,
Issue. 3,
p.
252.
Vidal, O.
and
Parra, T.
2000.
Exhumation paths of high‐pressure metapelites obtained from local equilibria for chlorite–phengite assemblages.
Geological Journal,
Vol. 35,
Issue. 3-4,
p.
139.