 )-exchanged bentonites upon thermal treatment
)-exchanged bentonites upon thermal treatmentPublished online by Cambridge University Press: 01 January 2024
Bentonites are excellent materials for sequestering various metal cations because of the cation fixation ability of the constituent montmorillonite layers, but sometimes, such as in the case of Cu2+, the exact location of cation fixation with respect to the clay layers is difficult to determine. Na-montmorillonite was prepared from the <2 µm fraction of the bentonite Calcigel (from Bavaria, Germany) and exchanged by Cu2+ and Na+ or by Cu2+ and NH4+\$\end{document} cations. The resulting materials (bi-ionic Cu-Na and Cu-NH4 samples, respectively, as well as homo-ionic forms with Cu2+, Na+ and NH4+\$\end{document}
cations. The resulting materials (bi-ionic Cu-Na and Cu-NH4 samples, respectively, as well as homo-ionic forms with Cu2+, Na+ and NH4+\$\end{document} ) were heated for 24 h at temperatures of 300 and 450°C and the structural evolution characterized using X-ray diffraction (XRD) analysis, Fourier Transform Infrared (FTIR) spectroscopy, and differential scanning calorimetry (DSC) analysis.
) were heated for 24 h at temperatures of 300 and 450°C and the structural evolution characterized using X-ray diffraction (XRD) analysis, Fourier Transform Infrared (FTIR) spectroscopy, and differential scanning calorimetry (DSC) analysis.
The XRD patterns showed that the Cu sample and the Cu-Na sample have basal spacings of 12.5 Å. Upon heating at 300 and 450°C, the layers collapsed to 9.5 Å. In contrast, the d001 value in the NH4 sample and Cu-NH4 sample decreased to 10.0 Å and 10.2 Å, respectively, during the heat treatment. The Cu2+ ions migrated irreversibly into the montmorillonite structure.
For the NH4 and the Cu-NH4 samples, DSC analyses show that NH3 evolved at between 300 and 400°C though the octahedral sheet was not altered substantially by the H+ generated. Infrared spectra show that the bands of the Si-O and OH vibrations of all samples were changed upon heating due to the movement of the dehydrated cations into the hexagonal holes of the tetrahedral sheet. Apparently no Cu2+ was trapped in the octahedral sheet. In the case of the Cu-NH4 form, both Cu2+ fixation and de-ammonization occurred during the heat treatment. Other than maintaining the basal spacing, no effect of the presence of NH4+\$\end{document}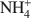 on the Cu2+ fixation could be found for the montmorillonite studied.
on the Cu2+ fixation could be found for the montmorillonite studied.