Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Koppelman, M. H.
and
Dillard, J. G.
1980.
Adsorption of Cr(NH3)63+ and Cr(en)33+ on Clay Minerals and the Characterization of Chromium by X-Ray Photoelectron Spectroscopy.
Clays and Clay Minerals,
Vol. 28,
Issue. 3,
p.
211.
Hall, Peter L.
1980.
The application of electron spin resonance spectroscopy to studies of clay minerals: II. Interlamellar complexes—structure, dynamics and reactions.
Clay Minerals,
Vol. 15,
Issue. 4,
p.
337.
Heller-Kallai, L
and
Yariv, S
1981.
Swelling of montmorillonite containing coordination complexes of amines with transition metal cations.
Journal of Colloid and Interface Science,
Vol. 79,
Issue. 2,
p.
479.
M.G. van den Berg, Constant
1984.
Determination of the complexing capacity and conditional stability constants of complexes of copper(II) with natural organic ligands in seawater by cathodic stripping voltammetry of copper-catechol complex ions.
Marine Chemistry,
Vol. 15,
Issue. 1,
p.
1.
Yamagishi, Akihiko
1987.
Optical Resolution and Asymmetric Syntheses by use of Adsorption on Clay Minerals.
Journal of Coordination Chemistry,
Vol. 16,
Issue. 2,
p.
131.
Ladavos, A.K.
and
Pomonis, P.J.
1991.
Preparation of Catalysts V - Scientific Bases for the Preparation of Heterogeneous Catalysts, Proceedings of the Fifth International Symposium.
Vol. 63,
Issue. ,
p.
319.
Puls, Robert W.
Powell, Robert M.
Clark, Donald
and
Eldred, Cynthia J.
1991.
Effects of pH, solid/solution ratio, ionic strength, and organic acids on Pb and Cd sorption on kaolinite.
Water, Air, and Soil Pollution,
Vol. 57-58,
Issue. 1,
p.
423.
Kijima, Tsuyoshi
Sakaguchi, Kenji
and
Ohe, Kaoru
1994.
Intercalation of [Pt(en)2]2+ and [PtCl2(en)2]2+ Ions by Na-Montmorillonite.
Bulletin of the Chemical Society of Japan,
Vol. 67,
Issue. 5,
p.
1281.
Fitch, Alanah
Song, John
and
Stein, Jenny
1996.
Molecular Structure Effects on Diffusion of Cations in Clays.
Clays and Clay Minerals,
Vol. 44,
Issue. 3,
p.
370.
Joo, Pal
Fitch, Alanah
and
Park, Sung-Ho
1997.
Transport in Hydrophobized Montmorillonite Thin Films.
Environmental Science & Technology,
Vol. 31,
Issue. 8,
p.
2186.
Bergaya, F.
and
Vayer, M.
1997.
CEC of clays: Measurement by adsorption of a copper ethylenediamine complex.
Applied Clay Science,
Vol. 12,
Issue. 3,
p.
275.
Fitch, Alanah
2002.
Access in Nanoporous Materials.
p.
93.
Ammann, L.
Bergaya, F.
and
Lagaly, G.
2005.
Determination of the cation exchange capacity of clays with copper complexes revisited.
Clay Minerals,
Vol. 40,
Issue. 4,
p.
441.
Gao, Hongsheng
Shori, Shailesh
Chen, Xiaoming
zur Loye, Hans-Conrad
and
Ploehn, Harry J.
2013.
Quantitative analysis of exfoliation and aspect ratio of calcium niobate platelets.
Journal of Colloid and Interface Science,
Vol. 392,
Issue. ,
p.
226.
Salem, Ibrahim A.
El-Ghamry, Hoda A.
and
El-Ghobashy, Marwa A.
2015.
Application of montmorillonite–Cu(II)ethylenediamine catalyst for the decolorization of Chromotrope 2R with H2O2 in aqueous solution.
Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy,
Vol. 139,
Issue. ,
p.
130.
Johnston, C. T.
and
Aochi, Y. O.
2018.
Methods of Soil Analysis.
p.
269.
Song, Nai-Meng
Yang, Li
Han, Ji-Min
Liu, Jian-Chao
Zhang, Guo-Ying
and
Gao, Hong-Xu
2018.
Catalytic study on thermal decomposition of Cu-en/(AP, CL-20, RDX and HMX) composite microspheres prepared by spray drying.
New Journal of Chemistry,
Vol. 42,
Issue. 23,
p.
19062.
Li, Zhaohui
Chang, Po-Hsiang
and
Jiang, Wei-Teh
2019.
Mechanisms of Cu2+, triethylenetetramine (TETA), and Cu-TETA sorption on rectorite and its use for metal removal via metal-TETA complexation.
Journal of Hazardous Materials,
Vol. 373,
Issue. ,
p.
187.
Song, Naimeng
Liu, Jianchao
Yang, Li
and
Liu, Pingan
2020.
Preparation of Nano‐Spherical Cu‐en and Its Catalytic Study on the Performance of Solid Propellant.
Propellants, Explosives, Pyrotechnics,
Vol. 45,
Issue. 11,
p.
1799.
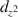 orbital is perpendicular to the clay surface. The montmorillonite particles may act as ligands and coordinate with Cu(en)22+ ions.
orbital is perpendicular to the clay surface. The montmorillonite particles may act as ligands and coordinate with Cu(en)22+ ions.