Published online by Cambridge University Press: 28 February 2024
The structure of montmorillonite intercalated with [Al13O4(OH)24+x(H2O)12−x](7−x)+ cations (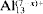 for short), where x = 0, 2 and 4, has been studied using the Cerius2 modeling environment. The Crystal Packer module used in the present study takes into account only the nonbonded interactions between the silicate layer and the Keggin cations. Minimization of the total sublimation energy led to the following conclusions: the structure of the interlayer (that is, the orientation of Keggin cations and the basal spacing) depends on the charge of cations (that is, on the degree of hydrolysis, x). The values of basal spacings in the range 19.38–20.27 Å have been obtained, depending on the charge and arrangement of cations in the interlayer. The dominating contribution to the total sublimation energy comes from the electrostatic interactions. Translations of
for short), where x = 0, 2 and 4, has been studied using the Cerius2 modeling environment. The Crystal Packer module used in the present study takes into account only the nonbonded interactions between the silicate layer and the Keggin cations. Minimization of the total sublimation energy led to the following conclusions: the structure of the interlayer (that is, the orientation of Keggin cations and the basal spacing) depends on the charge of cations (that is, on the degree of hydrolysis, x). The values of basal spacings in the range 19.38–20.27 Å have been obtained, depending on the charge and arrangement of cations in the interlayer. The dominating contribution to the total sublimation energy comes from the electrostatic interactions. Translations of 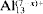 cations along the 2:1 layers give only small fluctuations of the total sublimation energy and basal spacings. No preference for the position of
cations along the 2:1 layers give only small fluctuations of the total sublimation energy and basal spacings. No preference for the position of 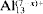 cations in the interlayer of montmorillonite was found during translation along the 2:1 layers. This result confirmed the inhomogeneous distribution of cations in the interlayer and turbostratic stacking of layers.
cations in the interlayer of montmorillonite was found during translation along the 2:1 layers. This result confirmed the inhomogeneous distribution of cations in the interlayer and turbostratic stacking of layers.