Published online by Cambridge University Press: 02 April 2024
The lacustrine Sucker Creek Formation (Miocene) of eastern Oregon includes unaltered vitric tuffs as well as tuffs altered to the following diagenetic fades: (1) bentonite, (2) interbedded bentonite and opal-CT, (3) K-clinoptilolite, and (4) Ca-clinoptilolite. Bentonite beds contain Fe-rich smectite (8–10 wt. % Fe2O3), quartz, plagioclase, and Ca-clinoptilolite. Opal-CT-rich layers contain inorganic silica (opal-CT), Fe-rich smectite, and minor diatoms. K-clinoptilolite beds typically contain clinoptilolite that can be extremely K-rich (≤7.6 wt. % K2O), opal-CT, smectite, plagioclase, and K-feldspar. This diagenetic facies also includes smectitic tuff and unaltered tuff. Ca-clinoptilolite beds contain Ca-clinoptilolite, quartz, K-feldspar, smectite, and illite.
Based on its chemistry and mineralogy, the bentonite appears to have been derived from dacitic volcanic ash. Chemical considerations and the close spatial relationship between beds of bentonite and opal-CT suggest that the diagenetic alteration of glass to smectite provided silica to the adjacent opal-CT beds. Based on the presence of late-stage Ca-clinoptilolite, alteration appears to have proceeded in a relatively closed chemical system.
Based on the composition of preserved vitric tuff, the zeolitic tuffs appear to be derived from rhyolitic ash, which diagenetically altered in an open hydrologic system and produced vertical zonations in mineralogy. In this model, bentonite horizons at the top of the K-clinoptilolite diagenetic fades formed by reaction of volcanic glass with dilute fluids that had a relatively low (Na+ + K+ + Ca2+)/H+ activity ratio and aH4SiO4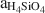 , whereas the underlying K-clinoptilolite beds formed from reactions between glass and dilute fluids having a higher (Na+ + K+ + Ca2+)/H+ activity ratio and aH4SiO4
, whereas the underlying K-clinoptilolite beds formed from reactions between glass and dilute fluids having a higher (Na+ + K+ + Ca2+)/H+ activity ratio and aH4SiO4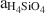 . Unaltered vitric ash between these beds may represent zones of higher permeability that inhibited secondary mineral alteration. Ca-clinoptilo-lite-rich beds appear to have undergone alteration similar to K-clinoptilolite-rich beds as well as to have been subjected to later, low-temperature (perhaps 75°–150°C) hydrothermal alteration which enhanced cation exchange in the zeolite and formed quartz from opal-CT.
. Unaltered vitric ash between these beds may represent zones of higher permeability that inhibited secondary mineral alteration. Ca-clinoptilo-lite-rich beds appear to have undergone alteration similar to K-clinoptilolite-rich beds as well as to have been subjected to later, low-temperature (perhaps 75°–150°C) hydrothermal alteration which enhanced cation exchange in the zeolite and formed quartz from opal-CT.
Deceased, August 19, 1989.
Succor Creek refers to the stream that flows through the east-central Oregon area, and Sucker Creek Formation refers to the geologic formation described in this article.