Published online by Cambridge University Press: 01 January 2024
Large transition-metal contents add desirable physical properties, such as redox reactivity, magnetism, and electric or ionic conductivity to micas and make them interesting for a variety of materials-science applications. A Mn- and F-rich tainiolite mica,  , was synthesized by a high-temperature melt-synthesis technique. Subsequent annealing for 10 days led to a single-phase and coarsegrained material. Single-crystal X-ray diffraction studies were performed and characteristic geometric parameters were compared to the analogous ferrous compound, synthetic Fe-rich tainiolite,
, was synthesized by a high-temperature melt-synthesis technique. Subsequent annealing for 10 days led to a single-phase and coarsegrained material. Single-crystal X-ray diffraction studies were performed and characteristic geometric parameters were compared to the analogous ferrous compound, synthetic Fe-rich tainiolite, 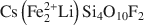 . Both tainiolite structures are outside the compositional stability limits for the 2:1 layer structure, and incorporating the relatively large cation Mn2+ requires significant structural adjustments in both the octahedral and tetrahedral sheets. As expected, increasing the ionic radius of the octahedral cation from 0.78 Å (VIFe2+) to 0.83 Å (VIMn2+) reduces the octahedral flattening angle from <Ψ> = 57.05° to <Ψ> = 56.4°, the smallest value ever observed for a tetrasilicic mica. However, even this small <Ψ> value is insufficient to match the lateral sizes of the tetrahedral and octahedral sheets and, in addition, unusual structural adjustments in the tetrahedral sheet are required. The average tetrahedral bond length <T-O> is much greater (1.643 Å) than the average value observed for tetrasilicic micas (1.607 Å,) and a significant difference between the <T-O>apical (1.605 Å) and the <T-O>basal bond lengths (1.656 Å) and an enlarged basal flattening angle (τbas = 106.29°) are noted. These parameters indicate: (1) that the 2:1 layer might be more flexible than previously thought, to allow matching of the lateral dimensions of the tetrahedral and octahedral sheets; and (2) that many other compositions that appear interesting from a materials-science point of view might be accessible.
. Both tainiolite structures are outside the compositional stability limits for the 2:1 layer structure, and incorporating the relatively large cation Mn2+ requires significant structural adjustments in both the octahedral and tetrahedral sheets. As expected, increasing the ionic radius of the octahedral cation from 0.78 Å (VIFe2+) to 0.83 Å (VIMn2+) reduces the octahedral flattening angle from <Ψ> = 57.05° to <Ψ> = 56.4°, the smallest value ever observed for a tetrasilicic mica. However, even this small <Ψ> value is insufficient to match the lateral sizes of the tetrahedral and octahedral sheets and, in addition, unusual structural adjustments in the tetrahedral sheet are required. The average tetrahedral bond length <T-O> is much greater (1.643 Å) than the average value observed for tetrasilicic micas (1.607 Å,) and a significant difference between the <T-O>apical (1.605 Å) and the <T-O>basal bond lengths (1.656 Å) and an enlarged basal flattening angle (τbas = 106.29°) are noted. These parameters indicate: (1) that the 2:1 layer might be more flexible than previously thought, to allow matching of the lateral dimensions of the tetrahedral and octahedral sheets; and (2) that many other compositions that appear interesting from a materials-science point of view might be accessible.