Published online by Cambridge University Press: 01 January 2024
A calorimetric method for determining isothermal partial and integral heats of hydration reactions (ΔH¯R,T,P${\rm{\Delta }}{\bar H_{{\rm{R,}}T,\,P}}$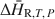 and ΔH∼R,T,P${\rm{\Delta }}{\tilde H_{{\rm{R,}}T,\,P}}$
and ΔH∼R,T,P${\rm{\Delta }}{\tilde H_{{\rm{R,}}T,\,P}}$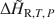 , respectively) in zeolites and other mineral hydrates is presented. The method involves immersing a dehydrated sample in a humid gas stream under isothermal conditions within a thermal analysis device that records simultaneous differential scanning calorimetric (DSC) and thermogravimetric analysis (TGA) signals. Monitoring changes in sample mass (corresponding to extent of reaction progress) coincident with a quantitative measurement of heat flow allows for direct detection of ΔH¯R,T,P${\rm{\Delta }}{\bar H_{{\rm{R,}}T,\,P}}$
, respectively) in zeolites and other mineral hydrates is presented. The method involves immersing a dehydrated sample in a humid gas stream under isothermal conditions within a thermal analysis device that records simultaneous differential scanning calorimetric (DSC) and thermogravimetric analysis (TGA) signals. Monitoring changes in sample mass (corresponding to extent of reaction progress) coincident with a quantitative measurement of heat flow allows for direct detection of ΔH¯R,T,P${\rm{\Delta }}{\bar H_{{\rm{R,}}T,\,P}}$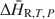 as a function of the extent of hydration, which can be integrated to determine ΔH∼R,T,P${\rm{\Delta }}{\tilde H_{{\rm{R,}}T,\,P}}$
as a function of the extent of hydration, which can be integrated to determine ΔH∼R,T,P${\rm{\Delta }}{\tilde H_{{\rm{R,}}T,\,P}}$ . In addition, it eliminates uncertainties associated with imprecise knowledge of the starting and final states of a sample during hydration. Measurement under isothermal conditions removes uncertainties associated with heat capacity effects that complicate interpretations of DSC measurements of dehydration heats conducted under traditional scanning temperature conditions. Example experiments on the zeolites natrolite, analcime and chabazite are used to illustrate strategies for quantifying ΔH¯R,T,P${\rm{\Delta }}{\bar H_{{\rm{R,}}T,\,P}}$
. In addition, it eliminates uncertainties associated with imprecise knowledge of the starting and final states of a sample during hydration. Measurement under isothermal conditions removes uncertainties associated with heat capacity effects that complicate interpretations of DSC measurements of dehydration heats conducted under traditional scanning temperature conditions. Example experiments on the zeolites natrolite, analcime and chabazite are used to illustrate strategies for quantifying ΔH¯R,T,P${\rm{\Delta }}{\bar H_{{\rm{R,}}T,\,P}}$ and ΔH∼R,T,P${\rm{\Delta }}{\tilde H_{{\rm{R,}}T,\,P}}$
and ΔH∼R,T,P${\rm{\Delta }}{\tilde H_{{\rm{R,}}T,\,P}}$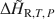 and minimizing errors associated with baseline uncertainties. Results from this method agree well with previously published values determined by other calorimetric techniques and regression of phase equilibrium data. In the case of chabazite, the results allowed detailed measurements of the variation in ΔH¯R,T,P${\rm{\Delta }}{\bar H_{{\rm{R,}}T,\,P}}$
and minimizing errors associated with baseline uncertainties. Results from this method agree well with previously published values determined by other calorimetric techniques and regression of phase equilibrium data. In the case of chabazite, the results allowed detailed measurements of the variation in ΔH¯R,T,P${\rm{\Delta }}{\bar H_{{\rm{R,}}T,\,P}}$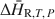 for energetically different water types encountered progressively as the sample absorbed water. This technique complements and in many cases improves the quality of thermodynamic data obtained through phase equilibrium observations and other calorimetric techniques.
for energetically different water types encountered progressively as the sample absorbed water. This technique complements and in many cases improves the quality of thermodynamic data obtained through phase equilibrium observations and other calorimetric techniques.