Article contents
Diagenetic Illite-Chlorite Assemblages in Arenites. II. Thermodynamic Relations
Published online by Cambridge University Press: 28 February 2024
Abstract
The internal equilibrium status among chlorite-illite pairs has been evaluated through coupled substitution reactions. Compositional data of chlorites and illites from arenites at present burial temperatures between 90° and 180°C have been used to calculate end member activities and reaction quotients of the combined Tschermak reaction: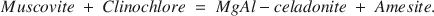
The reaction quotient data in the 90° to 180°C temperature range have then been compared with the equilibrium curve for the same reaction, and found to be in reasonable agreement. This indicates that chlorites and illites in these arenites grow at near equilibrium conditions.
The data set has also been compared with chlorite-illite pairs from hydrothermally altered arenites of the Salton Sea area. For chlorite-illite containing assemblages, these data agree well with the diagenetic ones. The introduction of biotite at higher temperatures alters with the iron-magnesium distribution and breaks down the substitution relationship between chlorite and illite.
The model predicts an increasing stability of muscovite and clinochlore components with increasing temperature, while celadonite and amesite would be stabilized with increasing pressure. This is consistent with high pressure occurrences of phengite. However, at the low pressure region of diagenesis and hydrothermal alteration, the temperature effect is dominant.
- Type
- Research Article
- Information
- Copyright
- Copyright © 1992, The Clay Minerals Society
References
- 12
- Cited by


