Published online by Cambridge University Press: 01 January 2024
Chrysotile from Thetford Mines in Quebec, Canada was treated first with mild formic or oxalic acid at concentrations of 0.5 to 2.0 N at 200°C in Teflon-lined 12.0 mL Parr bombs. The reaction products were identified by X-ray diffraction as a poorly crystalline Fe-bearing kerolite-like 2:1 layer silicate (which will be described as a kerolitic precipitate or a kerolitic mesophase in this report). Electron microscopic examination showed a thin foily morphology for this kerolitic mesophase that may have formed by the following reaction:(1)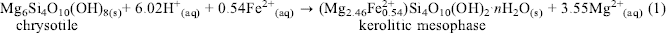
The magnetite impurity in the initial chrysotile asbestos served as the source of Fe in the above reactions. Subsequently, this kerolitic precipitate was reacted with 0.2 N NaOH for 48–96 h at 200°C and a highly crystalline smectite was formed with the same foily morphology as the kerolitic precipitate. X-ray spectral analyses of the kerolitic mesophase and smectite suggest the following reaction to have taken place:(2)
The reaction products, a kerolitic mesophase and smectite, possess a non-fibrous habit in contrast to the fibrous (asbestiform) morphology of chrysotile.