Introduction
Enzymes are efficient biocatalysts which enhance the reaction rates of various biological and chemical processes (Madhavan et al., Reference Madhavan, Sindhu, Binod, Sukumaran and Pandey2017). Lipase (EC 3.1.1.3) is one of the most important industrial enzymes and has the ability to catalyze a wide range of reactions in non-aqueous and aqueous environments (Aghaei et al., Reference Aghaei, Ghavi, Hashemkhani and Keshavarz2020), e.g. hydrolysis (Akash & Weatherley, Reference Akash and Weatherley2018; Atiroğlu, Reference Atiroğlu2020; Urrutia et al., Reference Urrutia, Arrieta, Alvarez, Cardenas, Mesa and Wilson2018), acidolysis (de Paula et al., Reference de Paula, dos Santos, Nunes and de Castro2018; Kavadia et al., Reference Kavadia, Yadav, Odaneth and Lali2018), transesterification (Kołodziejska et al., Reference Kołodziejska, Studzińska and Pawluk2018; Löfgren et al., Reference Löfgren, Görbe, Oschmann, Svedendahl Humble and Bäckvall2019; Wijnants et al., Reference Wijnants, Cornet and Bauwelinck2018), and esterification (Barse et al., Reference Barse, Graebin, Cipolatti, Robert, Pinto and Pinto2019; de Barros et al., Reference de Barros, Fernandez-Lafuente, Aguieiras and Freire2019; Papadaki et al., Reference Papadaki, Fernandes, Guimaraes Freire, Koutinas, Cavalcanti da Silva and Fernandez-Lafuente2018). Some lipase properties are often unavailable for industrial applications because the catalytic efficiency and stability of natural lipase are limited by various environmental factors, including high temperature, extreme pH (strong acid or alkaline), high salinity, and organic solvents (Madhavan et al., Reference Madhavan, Sindhu, Binod, Sukumaran and Pandey2017). Immobilization has proven to be a powerful tool to improve many features of lipase and to solve the problems of recovery and stability which have limited the application of enzymes as industrial catalysts (Urrutia et al., Reference Urrutia, Arrieta, Alvarez, Cardenas, Mesa and Wilson2018). The interfacial activation of lipase is a key factor in lipase immobilization. As reported by Brzozowski et al. (Reference Brzozowski, Derewenda, Derewenda, Dodson, Lawson and Turkenburg1991), the active center of the lipase molecule is covered by a movable region known as the “lid.” When lipase is adsorbed on the hydrophobic supports, the hydrophobic microenvironment on the carrier exposes the lid completely (Manoel et al., Reference Manoel, Dos Santos, Freire, Rueda and Fernandez-Lafuente2015), causing improvement in enzymatic activity.
Various methods have been proposed for the immobilization of lipase. The main strategies can be divided into physical methods and chemical methods. The physical methods include adsorption and entrapment in which the interaction between matrix and enzyme is weak, but the conformation of the enzyme can be retained (Sheldon, Reference Sheldon2007). The chemical methods refer to the immobilization of the enzyme on the modified support via covalent binding or cross-linking (Hanefeld et al., Reference Hanefeld, Gardossi and Magner2009). Sometimes, functional groups on the support material are activated by certain reagents to provide multipoint sites for the enzyme, which improves its stability.
The immobilization of the enzyme not only improves its stability, but also its catalytic activity, and also reduces its inhibition by substrates or products. These advantages depend on the lipase itself, on the immobilization technique, and on the support (Cipolatti et al., Reference Cipolatti, Manoel, Fernandez-Lafuente and Freire2017; Fernandez-Lafuente et al., Reference Fernandez-Lafuente, Armisén, Sabuquillo, Fernández-Lorente and Guisán1998; Palomo et al., Reference Palomo, Femandez-Lorente and Mateo2007). Among these, the support is an important factor in determining the effectiveness of the immobilized enzyme. Immobilization of lipase on hydrophobic supports has been attributed to the interfacial activation of the lipase vs the support surface (Fernandez-Lafuente et al., Reference Fernandez-Lafuente, Armisén, Sabuquillo, Fernández-Lorente and Guisán1998). As a general rule, a more hydrophobic support may immobilize more lipase than a less hydrophobic one.
The ideal support for enzyme immobilization should have excellent biocompatibility, stable physical and chemical properties, and abundant binding sites for the enzyme (Hartmann & Kostrov, Reference Hartmann and Kostrov2013). The most commonly employed supports include nanofibers, polymeric monoliths, mesoporous materials, nanomaterials, membranes, and cellulose paper. In recent years, nano-materials have provided new opportunities for the construction of nanostructured enzyme catalysts due to their large specific surface area, significant surface reactivity, and great mechanical strength (Wang et al., Reference Wang, Sun, Ma, Dong, Huo and Li2019). Previous research suggested that the Candida antarctica lipase B (CALB) immobilized on poly (glycidyl methacrylate-co-methylacrylate)/feather polypeptide (P(GMA-coMA)/FP) nanofibrous membranes that contain reactive epoxy groups and biocompatible FP preserved ~38% of the catalytic activity after treatment at 70°C for 3 h (Liu et al., Reference Liu, Chen and Shi2018a, Reference Liu, Fang, Yang, Li and Wangb). Another study (Wang et al., Reference Wang, Zhao and Yu2016) showed that a magnetic support consisting of carboxyl-functionalized Fe3O4 nanorods and MIL-100 (Fe) was employed to immobilize Candida rugosa lipase (CRL), and ~60% of the initial activity of CRL was retained even after ten reaction cycles (Wang et al. Reference Wang, Zhao and Yu2016). New functional materials integrating the benefits of large porosity, simple operation, tunable morphology, and ultra-large surface area:volume ratios are anticipated.
The amino organophyllosilicates (amino-clays), which belong to the family of inorganic-organic hybrid materials, possess an array of organic moieties within an inorganic matrix to form a 2:1 trioctahedral talc-like structure (Burkett et al., Reference Burkett, Press and Mann1997). The prime advantage of amino-clay is that it offers free and reversible exfoliation in water by protonation, but reverts easily to the stacked form by the addition of less-polar solvents such as ethanol (Datta et al., Reference Datta, Achari and Eswaramoorthy2013). Amino-clay has been employed widely, therefore, as a carrier for the immobilization of biomolecules including DNA (Patil et al., Reference Patil, Li, Dujardin and Mann2007), protein (myoglobin, haemoglobin), glucose oxidase (Patil et al., Reference Patil, Muthusamy and Mann2005), and anti-inflammatory drugs (ibuprofen, antioxidant polyphenol, and epigallocatechin) (Holmström et al., Reference Holmström, Patil, Butler and Mann2007). A previous study showed that the stabilities of the intercalated DNA and immobilized enzyme at elevated temperatures over a wide range of pH were improved significantly (Holmström et al., Reference Holmström, Patil, Butler and Mann2007). The catalytic efficiency of free lipase is very strict for pH, temperature, high salinity, etc. The aim of the current study was to improve the catalytic performance and stability of lipase by constructing a nanostructured lipase via Mg-amino-clay as a carrier.
Materials and Methods
Materials and Reagents
Lipase (from A. oryzae, lyophilized powder), bovine serum albumin (BSA), p-nitrophenyl phosphate (p-NPP), p-nitrophenol (p-NP), and 1-(3-Dimethylaminopropyl)-3-ethyl-carbodiimide hydrochloride (EDC) were supplied by Aladdin Industrial Corp. (Shanghai, China). 3-aminopropyltriethoxysilane (APTES) was purchased from Macklin Biochemical Co., Ltd. (Shanghai, China). All other chemicals, such as anhydrous ethanol, MgCl2·6H2O, Coomassie Brilliant Blue G-250, and tris (hydroxymethyl) methyl aminomethane hydrochloride (Tris-HCl) were acquired from Chengdu Kelon Chemical Reagent Company (Chengdu, China).
Characterization
The morphology of the samples (Mg-amino-clay, Mg-clay-lipase) was characterized by scanning electron microscopy (SEM) (Sigma300, Carl Zeiss, Jena, Germany), where samples were mounted on double-sided adhesive tape and sputter coated with 5–10 nm of gold prior to imaging at an accelerating voltage of 5 kV. Mineralogy was determined by X-ray diffraction (XRD) (Ultima IV with D/teX Ultra, Rigaku, Tokyo, Japan) with a small-angle accessory from 2 to 25°2θ using CuKα radiation (λ = 1.5406 Å, 40 kV, 30 mA). Fourier-transform infrared (FTIR) spectra of KBr pressed pellets (sample:KBr ratio of 1:100) were recorded using a (Perkin Elmer) Spectrum One instrument (Waltham, Massachusetts, USA) in the spectral range of 4000–400 cm−1 at a resolution of 4 cm−1. The zeta (ζ) potential was measured using a Zetasizer Nano ZS 90 (Malvern Instruments, Malvern, Worcestershire, UK). The UV-Vis absorption spectra were collected using a NanoDrop 2000C spectrophotometer (Thermo Scientific, Waltham, Massachusetts, USA).
Preparation of Mg-amino-clay
Ethanol (20 mL) was used to dissolve 8.4 g of MgCl2·6H2O (41.32 mmol), stirring at room temperature. 13 mL (58.73 mmol) of 3-aminopropyltriethoxysilane (APTES) was then added, drop-wise, with continuous stirring in a shaker. A white suspension was formed after several minutes, and stirring was continued overnight. The resulting products were centrifuged, washed with ethanol, and dried at 40°C. The white solid was then ground to produce a powder (Park et al., Reference Park, Lee and Hoang Bui2018).
Lipase Immobilization
To prepare Mg-clay-lipase, 0.1 g of Mg-amino-clay was reacted with 20 mL of a solution of EDC (0.4 g/L) in a phosphate buffer for 1 h at room temperature. In order to eliminate the remaining EDC, the precipitate was centrifuged and washed twice with a phosphate buffer (pH 7.0). After this, 0.1 g of the support was introduced into the solution of lipase (2.5 mg/mL), stirring for 3 h. The product was washed carefully with a phosphate buffer several times until all unbound lipase was removed and then used directly for the lipase activity measurements. The immobilization procedure is illustrated in Fig. 1.
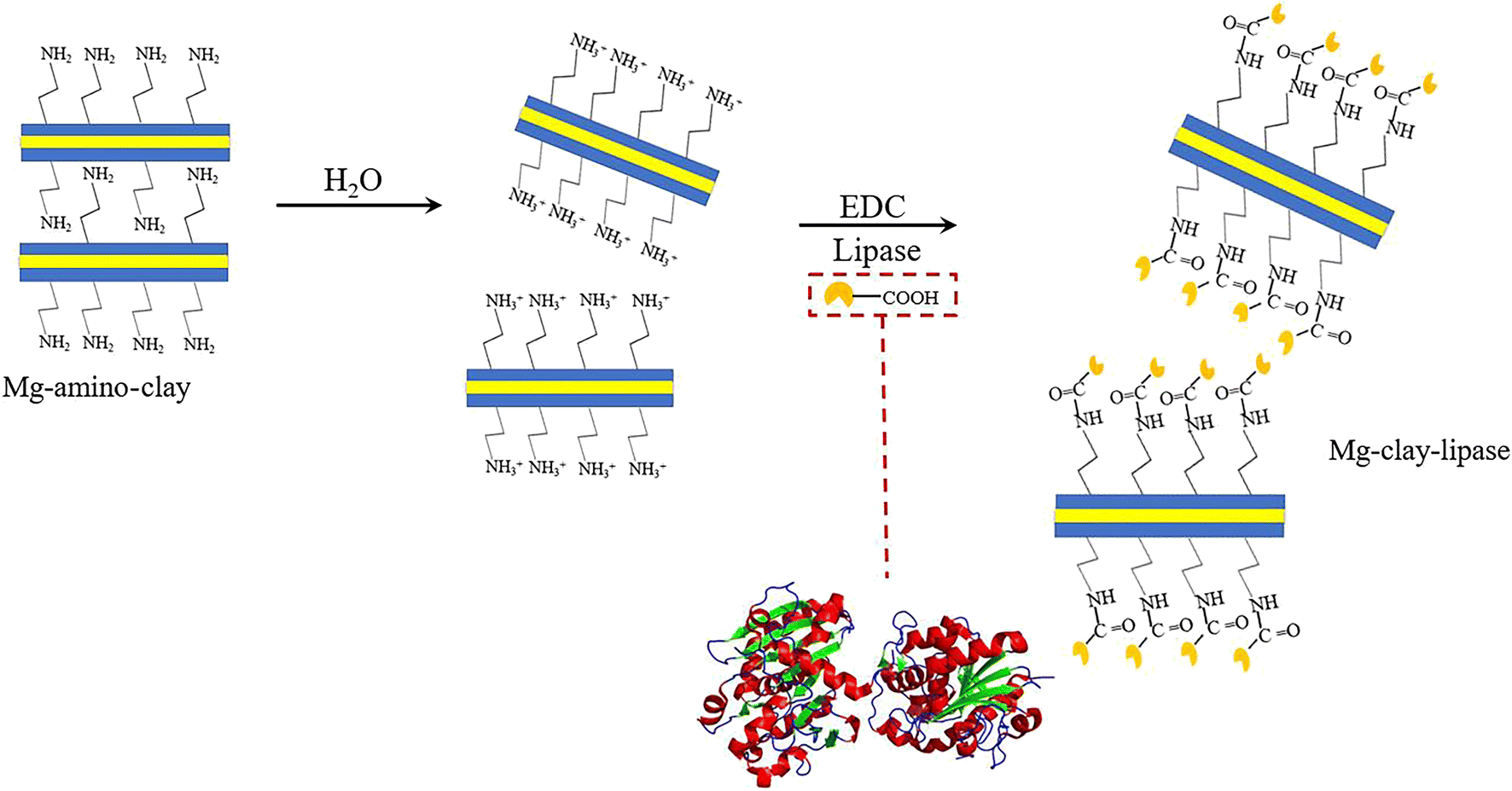
Fig. 1. Schematic diagram of Mg-amino-clay dispersed in water and lipase immobilization on Mg-amino-clay
Lipase Assay
The Bradford method was used to determine the amount of lipase loaded using BSA as a standard (Bradford, Reference Bradford1976). The amount of bound lipase was calculated indirectly by the difference between the lipase remaining in the supernatants and the amount of lipase loaded (Öztürk et al., Reference Öztürk, Pollet, Phalip, Güvenilir and Avérous2016). The lipase immobilization efficiency is given in Eq. 1.
where M 0 is the initial concentration of lipase solution and M 1 is the final unbound lipase concentration in the supernatant after immobilization.
Lipase Activity Assay
Hydrolysis of the p-nitrophenyl palmitate was investigated using a colorimetric method, as follows. The enzymatic activities of free lipase and Mg-clay-lipase were tested by hydrolysis of p-NPP (10 mM in 2-propanol) in Tris-HCl buffer (100 mM, pH 9) (Gupta et al., Reference Gupta, Jain and Jain2005). The reaction was initiated by adding free/immobilized lipase and incubated for 10 min at room temperature. The absorbance of the product (p-NP) was measured at 410 nm. Spontaneous hydrolysis of p-NPP was also considered using a blank, i.e. without lipase, as a control (Badoei-dalfard et al., Reference Badoei-dalfard, Malekabadi, Karami and Sargazi2019). One unit (U) of lipase was defined as the amount of enzyme that liberates one micromole of p-nitro phenol per minute under the assay conditions.
Kinetic Analysis
The dependence of enzyme activity on substrate concentration for the immobilized lipase on nanocomposites was evaluated by Michaelis-Menten (M-M) kinetics using Eq. 2. The kinetics of the free lipase and Mg-clay-lipase were studied by adding p-NPP as the substrate with increasing concentrations ranging from 0.2 to 4.0 mM.
where v max is the greatest possible specific lipase activity (U g−1), S is the concentration of substrate, and Km is the Michaelis-Menton (M-M) constant (mM) determined from the substrate concentration that gives a specific lipase activity of ½·v max. The kinetics of free lipase and Mg-clay-lipase were assayed for Km and the maximum reaction rate (v max), which were calculated from Lineweaver-Burk (Eq. 3) plots using the initial rate of the reaction, ν0, in which S 0 is the initial substrate concentration.
Results and Discussion
Characterization
SEM analysis
The micromorphologies of the various systems were examined to determine the structure and surface properties of the clay minerals. Scanning electron microscopy of the Mg-amino-clay (Fig. 2a) indicated that its morphology was of a uniformly layered structure, and the higher resolution image (Fig. 2b) showed lipase particles attached to a support, possibly by covalent immobilization, indicating that the lipase was loaded successfully onto the surface of the Mg-amino-clay.

Fig. 2. SEM images of a Mg-amino-clay and b Mg-clay-lipase
XRD and FTIR spectra analysis
Structurally, Mg-amino-clay is a type of 2:1 trioctahedral silicate (Wang et al., Reference Wang, Jing, Zhou and Lv2017). From XRD patterns of Mg-amino-clay and Mg-clay-lipase (Fig. 3a), the typical d 001 spacing of Mg-amino-clay was calculated to be 1.68 nm (5.25°2θ), which is typical for a layered organo clay (Park et al., Reference Park, Lee and Hoang Bui2018). The characteristic (d 020,110 and d 130,200) reflection at 22°2θ and the characteristic (d 060) reflection at 36°2θ confirmed the formation of the 2:1 trioctahedral Mg-phyllosilicate clay with a talc-like structure (Datta et al., Reference Datta, Achari and Eswaramoorthy2013). The characteristic reflection of the Mg-clay-lipase sample was the same as that of the Mg-amino-clay, so the layer spacing was unaffected by lipase adsorption, which confirmed that the distinctive structure of the Mg-amino-clay was retained. A likely reason was the intercalation of some amino acid residues of lipase while the polypeptide backbone of lipase remained outside of the Mg-amino-clay interlayer space (Sugunan & Gopinath, Reference Sugunan and Gopinath2007).
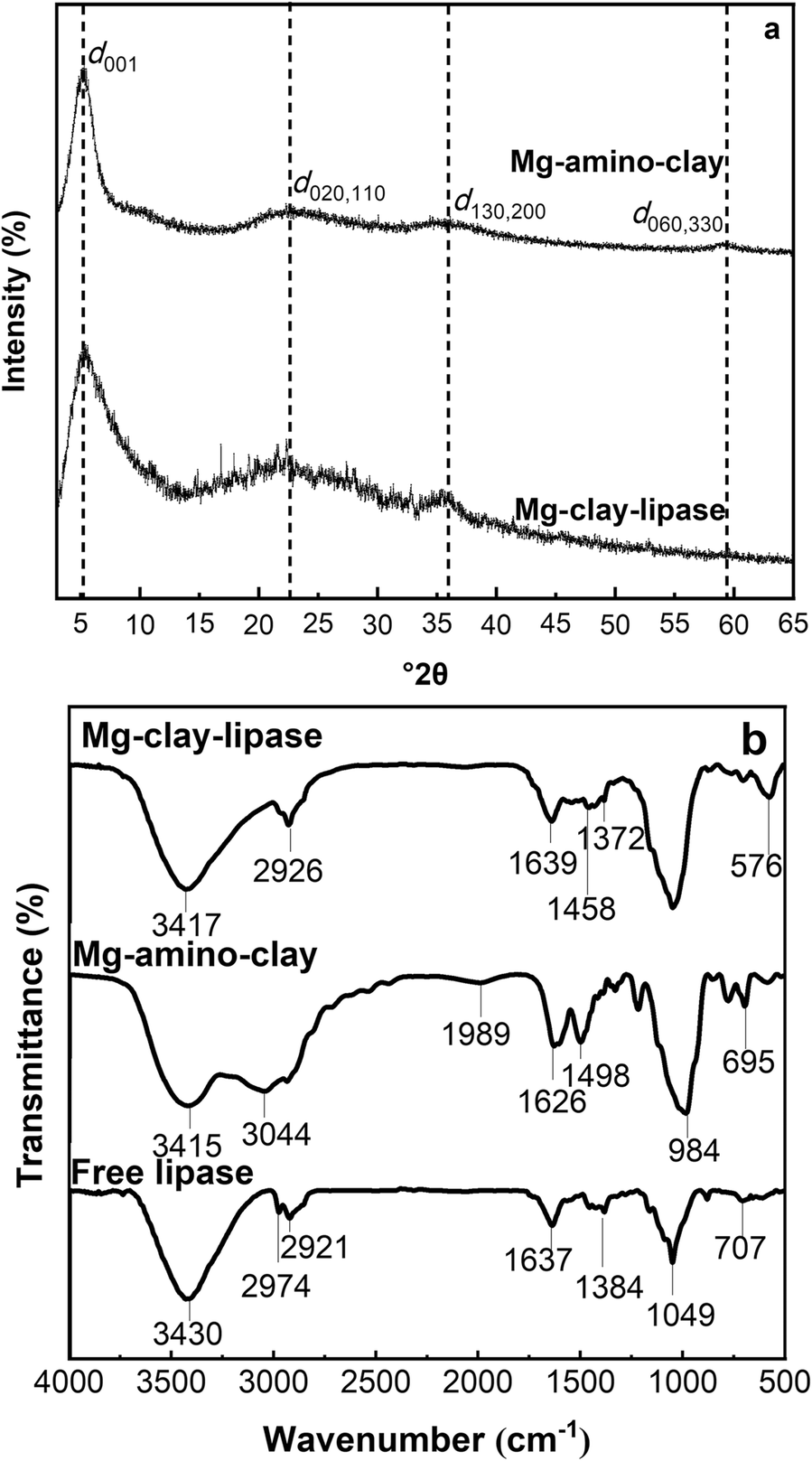
Fig. 3. a XRD patterns of Mg-amino-clay and Mg-clay-lipase, and b FTIR spectra of free lipase, Mg-amino-clay, and Mg-clay-lipase
FTIR spectroscopy is a universal technique for determining the secondary structures of proteins. The FTIR spectra of free lipase, Mg-amino-clay, and Mg-clay-lipase (Fig. 3b) provided evidence for the immobilization of lipase on Mg-amino-clay. The amide bond (–CO–NH–) consists of –COOH groups from lipase and –NH2 groups from the Mg-amino-clay layer (Patil et al., Reference Patil, Muthusamy and Mann2005), indicating a chemical (covalent) bond to immobilize the lipase on the Mg-amino-clay. Four absorption bands were characteristic of the Mg-amino-clay, at 984 cm−1 (Si–O–Si bending vibration), 1498 cm–1 (-CH2 bending vibration), 1626 cm–1 (–NH2 bending vibration), and 1989 cm−1 (–NH2 stretching vibration). Lipase showed three characteristic bands, at 3430 cm−1 (N–H stretching vibration), 2974 cm–1 and 2921 cm–1 (–CH2 stretching vibration), and 1637 cm−1 (C=O stretching vibration, which is also where the amide peak should appear) (Li et al., Reference Li, Li and Dong2012). The amide band at 1639 cm−1 also appeared in the spectrum of Mg-clay-lipase, confirming that the lipase had been immobilized on Mg-amino-clay via the covalent amide bond.
ζ potential analysis
Zeta potential is a significant parameter for reflecting the electrostatic interactions and stability of nanoparticles in aqueous solution. The larger the absolute value of ζ, the greater the dispersibility of the solution, so the system is more stable. Zeta potential analyses of free lipase, Mg-amino-clay, and Mg-clay-lipase under various pH values (Fig. 4) suggested that the potential of free lipase was negative, and that the absolute value increased with increase in pH. Mg-amino-clay had a positive potential because of the high density of -NH4 +, and the absolute value increased first and then decreased. ζ for Mg-clay-lipase was negative, and the absolute value increased with pH, indicating that the surface of Mg-amino-clay was loaded with negatively charged lipase, resulting in the absolute value of negative potential increasing. Mg-amino-clay showed more stable dispersion in aqueous solution compared with free lipase at pH 4 and pH 7. The reason for this was that a large amount of –NH4 + between the layers produced electrostatic repulsion after the Mg-amino-clay was stripped into a single layer in aqueous suspension (Park et al., Reference Park, Lee and Hoang Bui2018).

Fig. 4. Zeta (ζ) potential analysis of free lipase, Mg-amino-clay, and Mg-clay-lipase
Process for Immobilization of Lipase on Mg-amino-clay
The immobilization of lipase on Mg-amino-clay is generally considered to be by interfacial adsorption onto the amphiphilic surface of the clay, which may vary depending on adsorbate-adsorbate interactions. The relative catalytic activity of the Mg-clay-lipase increased as the amount of initial lipase increased up to 70 mg of lipase, and then increased no further above this amount (Fig. 5a). The leveling off must have been due to intermolecular interactions among lipase molecules as its density on the surface increased, thus preventing the exposure of further catalytic sites. 70 mg was, therefore, considered to be the optimum initial dosage of lipase. These results are consistent with other studies (Tudorache et al., Reference Tudorache, Coman and Protesescu2012; Liu et al., Reference Liu, Guo and Zhou2013).

Fig. 5. Optimization conditions for lipase immobilization on Mg-amino-clay: a the amount of initial lipase, b EDC concentration, and c reaction time
The effect of EDC concentration on the interaction of lipase and Mg-amino-clay was also measured (Fig. 5b). The results indicated that the EDC molecules were insufficient to activate –COOH for binding lipase below the optimum EDC concentration of 0.6 g/L. On the other hand, excessive use of EDC produced derivatives or released various functional groups, which changed the microenvironment around the carrier, thereby destroying the structure of the lipase or weakening the binding between lipase and the Mg-amino-clay.
The effect of varying reaction time from 1 h to 6 h on the interaction of lipase and Mg-amino-clay was examined further (Fig. 5c). The formation of amide groups between Mg-amino-clay and lipase was observed to be a time-consuming process, with the relative activity of Mg-clay-lipase peaking at 4 h. The relative activity of Mg-clay-lipase increased with reaction time up to 4 h and decreased afterwards. The cause was that the binding sites on Mg-amino-clay by lipase molecules reached saturation at 4 h. Enzyme activity decreased after this time due to denaturation. Finally, 4 h was chosen as the optimal immobilization time.
The enzymatic properties of Mg-clay-lipase prepared by EDC and the simple mixture of Mg-amino-clay and lipase under the optimal conditions were investigated to evaluate the effect of the amido bond between Mg-amino-clay and lipase (Fig. 6). The results suggested that the relative activity of free lipase was 74% of Mg-clay-lipase, while that of the simple mixture was only 22% of Mg-clay-lipase. The reason was that the lipase in the simple mixture was adsorbed on the Mg-amino-clay by electrostatic adsorption, which is very weak, resulting in a dramatic loss of lipase during centrifugation. On the other hand, the immobilized lipase adhered tightly to the surface of Mg-amino-clay by an amide bond with EDC.
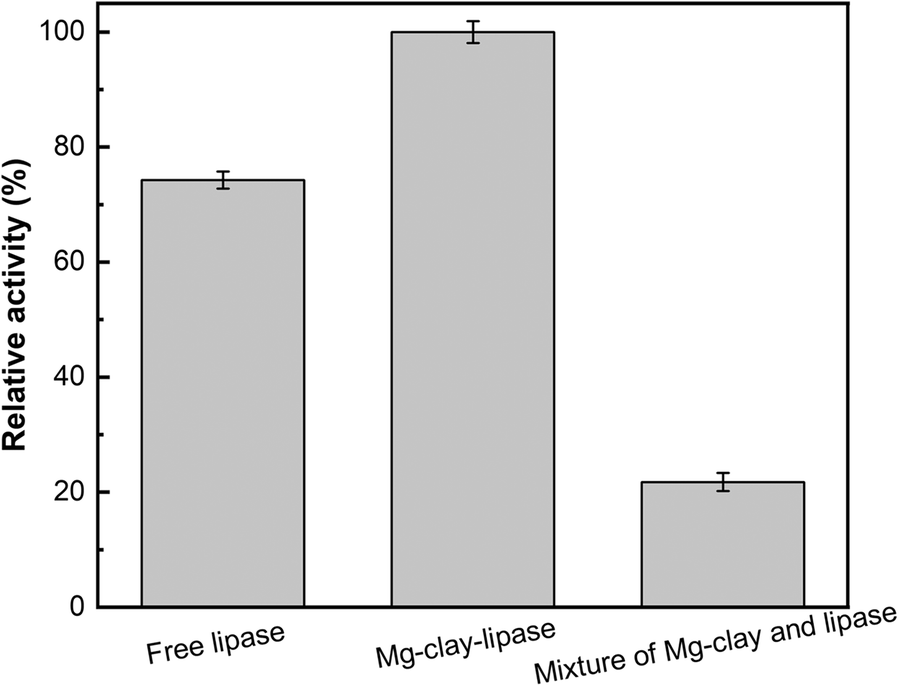
Fig. 6. Enzymatic activity of Mg-clay-lipase and of a mixture of Mg-amino-clay and lipase
Enzymatic Activities of Free Lipase and Mg-clay-lipase
Temperature is one of the parameters which affects enzymatic activity. The activities of free lipase and Mg-clay-lipase were evaluated in the range of ~20–60°C (Fig. 7a). The optimum temperature for Mg-clay-lipase was 10°C greater than that for free lipase. The reason was due to the carrier having an obvious effect on the conformational stability of lipase. Immobilization of lipase improved the rigidity of the three-dimensional structure and the stability of the lipase, reducing the influence of temperature on the lipase activity. Mg-clay-lipase required a greater activation energy to rearrange it into a suitable conformation and to form a lipase-substrate complex (Yong et al., Reference Yong, Bai, Li, Lin, Cui and Xia2008), so the optimal reaction temperature was necessarily greater. In addition, the heat energy of the substrate increased with the increase in temperature, and the collision rate of the substrate molecules with the enzyme also increased, causing the improvement in lipase activity as a result. Excessive temperature would destroy the advanced structure of the lipase protein, however, resulting in enzyme inactivation.
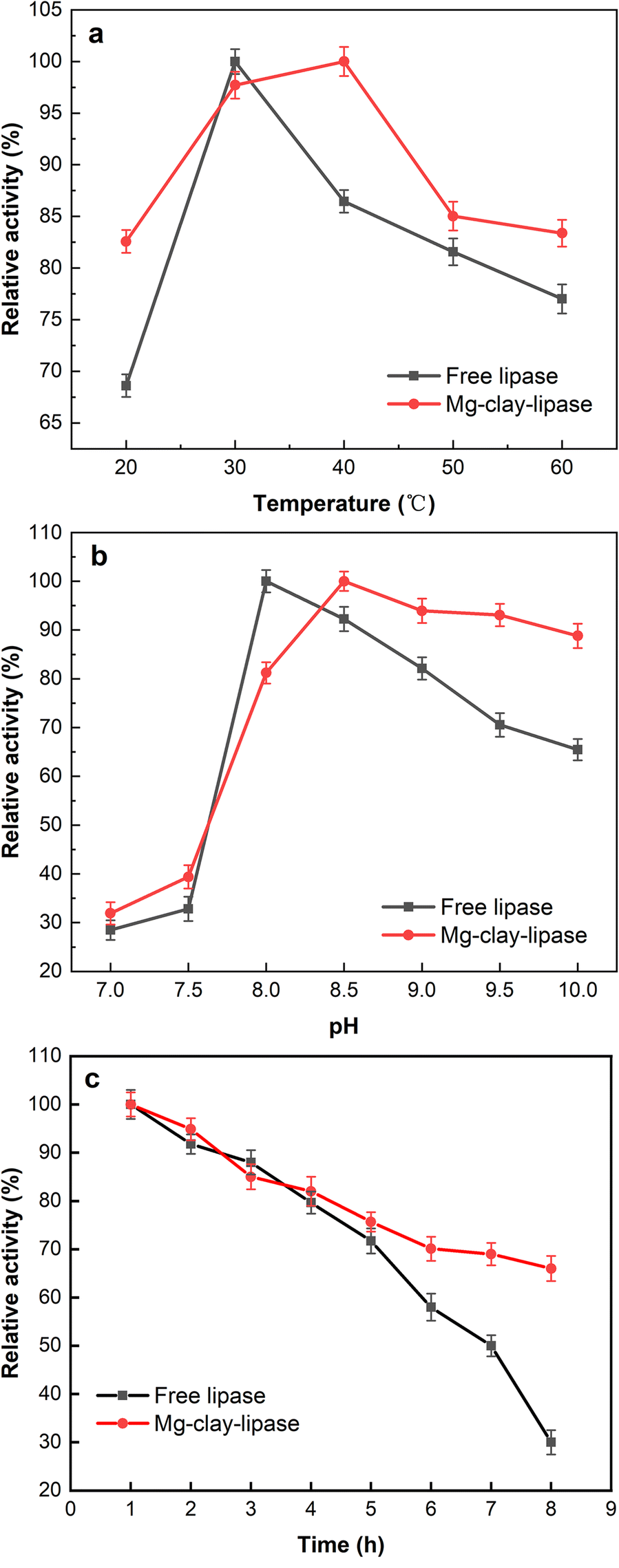
Fig. 7. Effect of a reaction temperature, b reaction pH, and c thermal stability at 60°C on enzymatic activity of free lipase and Mg-clay-lipase
pH is a critical parameter in enzymatic catalysis (Badoei-dalfard et al., Reference Badoei-dalfard, Malekabadi, Karami and Sargazi2019). The activities of free lipase and Mg-clay-lipase were examined from pH 7 to pH 10 (Fig. 7b). The optimum pH for free lipase was ~8.0, while that of Mg-clay-lipase was 8.5, and the stability of Mg-amino-clay was greater than that of free lipase. The higher optimum pH for Mg-clay-lipase was attributed to the anions and cations dissociated by the amino and carboxyl groups in Mg-clay-lipase, which played a role in regulating the acid-base balance of the reaction system, thus reducing the negative effect of a strong acid or base system on the structure of lipase.
The thermal stabilities of free lipase and Mg-clay-lipase were investigated after incubation at 60°C for 8 h (Fig. 7c). The results showed that the enzyme activity of free lipase preserved only ~30% of the initial activity in the 8th hour, while Mg-clay-lipase preserved 80% of the initial activity. This significant result was associated with the multipoint interactions between lipase and Mg-amino-clay which strengthened the intramolecular force and avoided the autolysis of lipase (Zhu et al., Reference Zhu, Zhang, Hou, Pan, He and Zhu2016).
A series of experiments revealed that the support was helpful for enzyme activity. Lipase is a special ester bond hydrolase with hydrophilic and hydrophobic ends. The active site of lipase was located at the hydrophobic end (Palomo et al., Reference Palomo, Femandez-Lorente and Mateo2007), covered by a “lid,” which is a movable region of the enzyme molecule. Interaction with a hydrophobic phase could lead to the opening of the lid to make the active site accessible; this phenomenon is referred to as “interfacial activation” (Brzozowski et al., Reference Brzozowski, Derewenda, Derewenda, Dodson, Lawson and Turkenburg1991; Liu et al., Reference Liu, Ma and Shi2020). Because interfacial activation could cause a dramatic increase in catalytic activity, the structure of lipase could be reasonably regulated by constructing a hydrophobic carrier or microenvironment so that the catalytic activity of lipase would be improved. The ATPES provided a large amount of –NH2 during the construction of the Mg-amino-clay, and the Mg-amino-clay would disperse into single layers in aqueous suspension. On the other hand, the hydrophobic alkane group (–CH2–) could make the Mg-amino-clay hydrophobic. When lipase was loaded onto the hydrophobic supports via covalent bonding, the hydrophobic microenvironment on the carrier made the lid stay open, leading to improvement in the enzymatic activity. As illustrated above, interface activation was the primary cause of enzymatic activity and of improving the tolerance of lipase to immobilization. The activity and thermostability of lipase could be changed by modifications of their lid domains (Rehm et al., Reference Rehm, Trodler and Pleiss2010). Furthermore, improving enzyme stability after immobilization could be ascribed to rigidification of enzyme conformation which inhibited protein unfolding (Mehrasbi et al., Reference Mehrasbi, Mohammadi, Peyda and Mohammadi2017).
The kinetic parameters of free lipase and Mg-clay-lipase were investigated by calculating initial reaction rates with different substrate concentrations. The kinetics of free lipase and Mg-clay-lipase were assayed for their Michaelis-Menten constant (Km) and maximum reaction rate (v max) (Table 1), which were calculated from Lineweaver-Burk plots using the initial rate of the reaction (Fig. 8). In the case of free lipase, the smaller Km value observed indicated a greater lipase affinity for the p-NPP. The main reason was that the presence of the carrier increased the steric hindrance around the lipase and increased the binding resistance between the substrate and the active center of the enzyme protein molecule on the carrier, thus reducing the affinity between the substrate and the enzyme and increasing the Km value. On the contrary, the maximum reaction rate, v max, of Mg-clay-lipase was greater than that of free lipase, indicating that Mg-clay-lipase had greater catalytic ability due to the increase in Mg-clay-lipase activity under the same conditions. To achieve the same catalytic effect, the Mg-clay-lipase required less time.
Table 1. Kinetic parameters of free lipase and Mg-clay-lipase
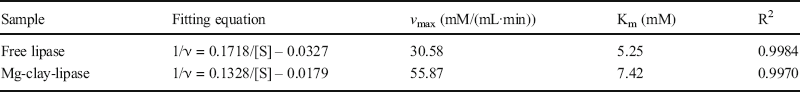

Fig. 8. Lineweaver-Burk plots of free lipase and Mg-clay-lipase
Conclusions
In the current study, a nanostructured lipase catalyst (Mg-clay-lipase) was synthesized by a covalent cross-linking method using talc-like magnesium amino clay (Mg-amino-clay) as the carrier. The optimal conditions for lipase immobilization included 70 mg of lipase, 0.6 g/L of EDC, and a reaction time of 4 h. The enzymatic activities assay indicated that the optimum reaction temperature of Mg-clay-lipase was 10°C higher than that of free lipase, and Mg-clay-lipase was more stable over a wide pH range; the thermal stability of Mg-clay-lipase was greater than that of free lipase under the optimal conditions. This research provided a new type of nanostructured lipase catalyst and infers that the excellent catalytic performance could be of great industrial value.
Acknowledgments
This study was funded by the National Natural Science Foundation of China (NSFC, grant number 42061134018, 42011530085), the Russian Foundation for Basic Research (RFBR, grant number 20-55-53019), and by the Sichuan Science and Technology Program (grant number 2019JDJQ0056).
Declarations
Conflict of Interest
The authors declare that they have no conflict of interest.











