Published online by Cambridge University Press: 28 February 2024
Consideration of XRD, TEM, AEM, and analytical data reported in the literature indicates that dioctahedral aluminous smectite and illite form two separate solid solutions that differ chemically from one another primarily by the extent of Al substitution for Si, the amount of interlayer K, and the presence of interlayer H2O. The data indicate that limited dioctahedral-trioctahedral and dioctahedral-vacancy compositional variations occur in both minerals. Excluding interlayer H2O and based on a half unit cell [i.e., O10(OH)2], natural dioctahedral smectite and illite solid solutions fall within the compositional limits represented by A0.3${\rm{R}}_{1.9}^{3 + }$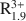 Si4O10(OH)2-AR2+ R3+ Si4O10(OH)2-A0.25${\rm{R}}_{0.3}^{2 + }{\rm{R}}_{1.8}^{3 + }$
Si4O10(OH)2-AR2+ R3+ Si4O10(OH)2-A0.25${\rm{R}}_{0.3}^{2 + }{\rm{R}}_{1.8}^{3 + }$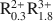 Al0.25Si3.75O10(OH)2 for smectites and A0.8${\rm{R}}_{1.9}^{3 + }$
Al0.25Si3.75O10(OH)2 for smectites and A0.8${\rm{R}}_{1.9}^{3 + }$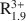 Al0.5Si3.5O10(OH)2-A0.55${\rm{R}}_{0.45}^{2 + }{\rm{R}}_{1.55}^{3 + }$
Al0.5Si3.5O10(OH)2-A0.55${\rm{R}}_{0.45}^{2 + }{\rm{R}}_{1.55}^{3 + }$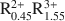 Al0.1Si3.9O10(OH)2-A0.9${\rm{R}}_{0.3}^{2 + }{\rm{R}}_{1.8}^{3 + }$
Al0.1Si3.9O10(OH)2-A0.9${\rm{R}}_{0.3}^{2 + }{\rm{R}}_{1.8}^{3 + }$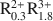 Al0.9Si3.1O10(OH)2 for illites, where A represents either monovalent cations or divalent cations expressed as their monovalent equivalent (e.g., Ca2+/2); R2+ stands for the divalent cations Mg2+ and Fe2+ and R3+ refers to the trivalent cations Al3+ and Fe3+. Taking account of these compositional limits, smectite and illite solid solutions can be described in terms of nine and six thermodynamic components, respectively, all of which are consistent with both the law of definite proportions and the concept of a unit cell. Thermodynamic components that can be used to describe natural smectite solid solutions in terms of a half unit cell [i.e., O10(OH)2] can be expressed as NaAl3Si3O10(OH)2, NaAl3Si3O10(OH)2 ·4.5H2O, Al2Si4O10(OH)2, Fe2Si4O10(OH)2, Mg3Si4O10(OH)2, Fe3Si4O10(OH)2, K3AlSi4O10(OH)2, KAl3Si3O10(OH)2, and Ca0.5Al3Si3O10(OH)2. Of these, NaAl3Si3O10(OH)2 ·4.5H2O provides explicitly for the presence of interlayer H2O in the mineral. Thermodynamic components representing illite solid solutions in natural systems can be written for a half unit cell as KAl3Si3O10(OH)2, KMg3AlSi3O10(OH)2, KFe3AlSi3O10(OH)2, Al2Si4O10(OH)2, KFe2AlSi3O10(OH)2, and K3AlSi4O10(OH)2. The calculations and observations summarized below indicate that neither smectite nor illite occur in nature as stoichiometric phases and that the two minerals do not form a mutual solid solution corresponding to mixed-layered illite/smectite.
Al0.9Si3.1O10(OH)2 for illites, where A represents either monovalent cations or divalent cations expressed as their monovalent equivalent (e.g., Ca2+/2); R2+ stands for the divalent cations Mg2+ and Fe2+ and R3+ refers to the trivalent cations Al3+ and Fe3+. Taking account of these compositional limits, smectite and illite solid solutions can be described in terms of nine and six thermodynamic components, respectively, all of which are consistent with both the law of definite proportions and the concept of a unit cell. Thermodynamic components that can be used to describe natural smectite solid solutions in terms of a half unit cell [i.e., O10(OH)2] can be expressed as NaAl3Si3O10(OH)2, NaAl3Si3O10(OH)2 ·4.5H2O, Al2Si4O10(OH)2, Fe2Si4O10(OH)2, Mg3Si4O10(OH)2, Fe3Si4O10(OH)2, K3AlSi4O10(OH)2, KAl3Si3O10(OH)2, and Ca0.5Al3Si3O10(OH)2. Of these, NaAl3Si3O10(OH)2 ·4.5H2O provides explicitly for the presence of interlayer H2O in the mineral. Thermodynamic components representing illite solid solutions in natural systems can be written for a half unit cell as KAl3Si3O10(OH)2, KMg3AlSi3O10(OH)2, KFe3AlSi3O10(OH)2, Al2Si4O10(OH)2, KFe2AlSi3O10(OH)2, and K3AlSi4O10(OH)2. The calculations and observations summarized below indicate that neither smectite nor illite occur in nature as stoichiometric phases and that the two minerals do not form a mutual solid solution corresponding to mixed-layered illite/smectite.
2 In accord with the classification system established by the AIPEA (Bailey et al.. 1984), the terms smectite and illite are used in the present communication to refer to two separate mineral groups that have specific crystallographic and chemical characteristics that distinguish the species in one group from those in the other.