Introduction
Uranium has been used widely for nuclear fuel and concerns have arisen about the recovery and the sustainable development of uranium salts, e.g. uranyl (UO2 2+). In general, the acidic wastewater of UO2 2+ originates from the development of uranium ore or the treatment of nuclear waste, which could undermine the acid-base balance of the constituent waters (Todorov & Ilieva Reference Todorov and Ilieva2006; Dhiraj et al. Reference Dhiraj, Garima and Kaur2008) and lead to diseases which endanger human health (Rufyikiri et al. Reference Rufyikiri, Wannijn, Wang and Thiry2006; Ai et al. Reference Ai, Liu, Lan, Jin, Xing and Zou2018). Currently, various methods of removing UO2 2+ from aqueous solutions are known; adsorption has been considered an effective method for UO2 2+ removal from wastewater or soil (Zhang et al. Reference Zhang, Wang, Li, Dai and Liu2013). Depending on the source of the pollutants, uraniferous wastewater may contain a considerable amount of toxic metal ions. The toxic trace-metal ions have a significant effect on the adsorption of UO2 2+ due to competition for sorption sites (Villalobos et al. Reference Villalobos, Trotz and Leckie2001). Take nuclear reactions as an example, some of the fission products are present as oxide precipitates (e.g. Rb+, Cs+, Ba2+, Zr4+) or solid solution forms in fuel matrices that are usually discharged with waste (Burns et al. Reference Burns, Ewing and Navrotsky2012). Likewise, uranium deposits often contain heavy metals (e.g. Cd2+, Cu2+, Hg2+, Pb2+, Zn2+, and As5+) that can be released into the environment during the mining process (Santos et al. Reference Santos, Barbosa, Pessoa, Leal and Reboredo2018).
Clay minerals are efficient adsorbents for toxic trace-metal ions because of their layered structure, large specific surface area, and large cation exchange capacity (Bedelean et al. Reference Bedelean, Măicăneanu, Burcă and Stanca2009). Clay minerals can also act as a matrix to produce selective clay adsorbents (Zhu et al. Reference Zhu, Chen, Zhou, Xi, Zhu and He2016; Hajjaji et al. Reference Hajjaji, Andrejkovicova, Pullar, Tobaldi, Lopez-Galindo, Jammousi, Rocha and Labrincha2016). For instance, Soliman et al. (Reference Soliman, Murad, Sheikh, Massad and Ali2017) reported an activated sludge from refinery wastewater with high selectivity for UO2 2+ ions in a mixture of Zr4+ , Cr3+ , Gd2+ , Sr2+ , Co2+ , Cs+, and Na+, and the capacity to adsorb UO2 2+ increased to 28 mg·g–1. Moreover, the competitive adsorption of Pb2+, Ni2+, Zn2+, Cd2+, and UO2 2+ in clay minerals (smectite and kaolinite) was measured to demonstrate the effect of soil constituents on adsorption (Choi & Park Reference Choi and Park2005). Montmorillonite, a traditional clay adsorbent, was applied in water treatment to remove metal ions (Bhattacharyya & Gupta Reference Bhattacharyya and Gupta2008). Campos et al. (Reference Campos, Aguilar-Carrillo, Algarra, Gonçalves, Rodríguez-Castellón and Silva2013) assessed the adsorption capacity of UO2 2+ onto Ca-montmorillonite as 8.57 mg·g–1, and only half of UO2 2+ was desorbed. The adsorption of UO2 2+ was achieved primarily on the surface of the montmorillonite, and the mechanism was split into ion exchange and the composite effect of the clay surfaces (McKinley et al. Reference McKinley, Zachara, Smith and Turner1995).
Montmorillonite cannot be recycled easily, and this reservation limits its application, though montmorillonite has excellent adsorption properties and available adsorption sites within its interlayer space and on the outer surface and edges. Recently, magnetic clay mineral nanocomposites have been used in the recovery of toxic and radioactive materials because of their low cost, low toxicity, and easy separation from solution under an external magnetic field (Lu et al. Reference Lu, Salabas and Schüth2010; Pylypchuk et al. Reference Pylypchuk, Kołodyńska, Kozioł and Gorbyk2016; Dorota et al. Reference Dorota, Marzena, Ievgen and Hubicki2016). Fe3O4-montmorillonite nanocomposite was synthesized to remove Pb2+, Cu2+, Cd2+, and Ni2+ ions from aqueous systems. The removal efficiency of Pb2+, Cu2+, and Ni2+ ranged from 76.15% to 94.89% in specific liquid-solid ratios (Kalantari et al. Reference Kalantari, Ahmad, Fard Masoumi, Shameli, Basri and Khandanlou2015), and the adsorption capacity of Cd2+ was 2 mg·g–1 (Tinas et al. Reference Tinas, Çalişkan, Özbek and Akman2016).
Montmorillonite modified with magnetic material is a potential selective adsorbent material. However, Fe3O4 nanoparticles exhibit a small particle size, so it is easier to agglomerate than other non-magnetic nanoparticles, resulting in the reduction of adsorption sites. The adsorption of uranium(VI) on magnetite (Fe3O4) nanoparticles was studied by Debasish et al. (Reference Debasish, Sureshkumar, Siddhartha, Mithal and Pillai2014); its adsorption capacity of 5 mg·g–1 was relatively low. The surface and material modifications of magnetic nanoparticles have been used extensively to enhance adsorption capacity and dispersion stability. The silica-coated magnetic Fe3O4 nanoparticles were modified with quercetin (Sadeghi et al. Reference Sadeghi, Azhdari, Arabi and Moghaddam2012), and the results revealed that the maximum monolayer adsorption capacity of UO2 2+ using the Langmuir isotherm was 12.33 mg·g–1. In addition, one of the modification methods to inhibit agglomeration and enhance adsorption capacity used transition metals as mediators to modify Fe3O4. Hu et al. (Reference Hu, Lo and Chen2005) investigated surface-modified jacobsite (MnFe2O4) nanoparticles. In their study, the maximum uptake of Cr(VI) was 31.5 mg·g–1 and the adsorption data fitted the Langmuir model well. As a result, the negatively charged montmorillonite could effectively adsorb cationic U(VI), and the surface-adsorbed Fe(II) could promote the reductive transformation of U(VI). The montmorillonite-modified zerovalent iron nanoparticles were synthesized by Hu et al. (Reference Hu, Ye and Ren2016) who then used it to remove radionuclides. Thus, the modified Fe3O4 based on montmorillonite was expected to have better applications in uranyl adsorption.
Functional magnetic nanoparticle/clay mineral nanocomposites have been used extensively recently (Zhou & Keeling Reference Zhou and Keeling2013; Chen et al. Reference Chen, Zhou and Fiore2016; Zhou et al. Reference Zhou, Zhao, Chen, Ai and Hong2016). In the present study, the goal was to synthesize MFe2O4 (M = Mn, Fe, Zn, Co, Ni)-montmorillonite nanoparticles through surface and material modification of Fe3O4, to test its ability to inhibit the aggregation of Fe3O4, and to characterize the structures using XRD, FTIR, Raman, TEM, SEM, and XPS. A further objective was to characterize the electronic activity of the interfaces using diffuse reflectance spectroscopy (DRS) and terahertz time-domain spectroscopy, and to use the adsorbents to remove U(VI) in the presence of toxic trace metal ions.
Materials and Methods
Materials
All the chemicals were of analytical grade. The montmorillonite (K-10) was purchased from Sigma-Aldrich (St. Louis, Missouri, USA), and the raw montmorillonite was determined to be montmorillonite-15A by XRD analysis (Supplementary Fig. S1). UO2(NO3)2•6H2O was purchased from Hubei Chushengwei Chemical Company (Hubei, China) and other reagents were purchased from ChengDu Chron Chemicals Reagent Company (Chengdu, China).
MFe2O4(M = Mn, Fe, Zn, Co, Ni)-montmorillonite was prepared using forced hydrolysis (Xuan et al. Reference Xuan, Zeng, Fan, Qin and Shu2010). Montmorillonite (3 g) was suspended in 200 mL of a mixed solution of 10 mmol MCl2·nH2O (M = Fe, Mn, Cu, Zn, Ni) and 20 mmol FeCl3·6H2O (molar ratio 1:2), with vigorous stirring for 0.5 h at room temperature. Under N2 gas atmosphere, the mixed solution was heated to 80°C and 2 M NaOH solution was added dropwise until pH = 11. Then the precursor was stirred continuously for 1 h at 80°C. After cooling to room temperature, the composite was centrifuged and washed repeatedly with distilled water and ethanol, then collected by magnetic separation. Finally, the samples were vacuum dried for 12 h at 60°C.
Characterization
The crystal structures of samples were determined using X-ray diffraction (XRD) equipped with a CuKα radiation source, over a scanning range of 3 to ~70°2θ (PANalytical B.V., Almelo, The Netherlands). The Fourier-transform infrared spectroscopy (FTIR) analysis was performed using a scanning range of 4000 to ~400 cm–1 (Perkin Elmer, Waltham, Massachusetts, USA). Raman scattering measurements were conducted under a backscattering geometric configuration using a confocal micro-Raman system (Renishaw, London, UK). The excitation light was at the 514.5 nm line for Ar+ laser, and at a maximum output of 30 mW. Scanning electron microscopy (SEM) of samples was performed using the Cu plate with a field emission machine (Carl Zeiss, Jena, Germany). Transmission electron microscopy (TEM) and high-resolution transmission electron microscopy (HRTEM) images were obtained using a Libra 200 system (Carl Zeiss, Jena, Germany). X-ray photoelectron spectroscopy (XPS) equipped with a DS300 unit was performed with a multifunctional electron spectrometer (Kratos, Manchester, UK) using monochromatic MgKα radiation to analyze the element distribution (Zou et al. Reference Zou, Song, Yi, Bian, Liu and Zhang2016). The photon information of the interfaces was tested by terahertz time-domain spectroscopy (THz-TDS) (Zomega, East Greenbush, New York, USA) (Wilke et al. Reference Wilke, Ramanathan, Lachance, Tamalonis, Aldersley and Joshi2014). UV-Vis diffuse reflectance spectra (DRS) (Shimadzu, Kyoto, Japan) (He et al. Reference He, Zong, Dong, Yang, Ke and Liu2017) and zeta potential measurement (Malvern, Worcestershire, UK) were utilized to investigate the electrophoretic mobility and electronic transition mechanism.
Competitive Adsorption
The uranyl-heavy metal mixed solution was prepared by combining 1.86 g of UO2(NO3)2·6H2O, 1.40 g of RbCl, 1.81 g of SrCl2, 5.13 g of CrCl3·6H2O, 2.29 g of MnCl2, 2.21 g of NiCl2, 2.08 g of ZnCl2, and 1.63 g of CdCl2, which were then dissolved completely in 1000 mL of deionized water to obtain a concentration of 1000 mg·L–1 for each metal ion. Aliquots of 0.5 mL, 1.0 mL, 1.5 mL, 2.0 mL, 2.5 mL, and 3.0 mL were taken from this mixed solution and diluted with deionized water to 50 mL to obtain mixed solutions having concentrations of 10 mg·L–1, 20 mg·L–1, 30 mg·L–1, 40 mg·L–1, 50 mg·L–1, and 60 mg·L–1 of UO2 2+-X n+ (X = Rb+, Sr2+, Cr3+, Mn2+, Ni2+, Zn2+ and Cd2+). The pH of the diluted solution was fixed at 5.5 using dilute hydrochloric acid, and 0.03 g MFe2O4(M = Mn, Fe, Zn, Co, Ni)-montmorillonite was added to the solution and then it was shaken mechanically for 24 h.
The residual concentration of uranyl-heavy metal was characterized using an inductively coupled plasma optical emission spectrometer (ICP-OES) (Perkin Elmer, Waltham, Massachusetts, USA) (Chen et al. Reference Chen, Wang, Zhao, Xiong, Cao and Dai2017). Adsorption behaviors were described by the Langmuir, Freundlich, and Dubinin-Radushkevich (D-R) isotherms. Therein, the Langmuir monolayer mode could describe the monolayer adsorption behaviors of the uniform adsorption sites (Langmuir Reference Langmuir1918), and the Freundlich mode was suitable for multilayer adsorption or non-ideal adsorption of heterogeneous active sites (Freundlich Reference Freundlich1906). The Dubinin-Radushkevich mode was taken to estimate the characteristic porosity and the apparent energy of adsorption (Dubinin Reference Dubinin1960). The corresponding isotherms were estimated using the following equation:
where q e is the equilibrium adsorption capacity of metal ion, C e is the equilibrium concentration (mmol·L–1), q max is the maximum adsorption capacity (mmol·g–1), and b is the adsorption equilibrium coefficient (L·mmol–1). kF and n are Freundlich parameters related to adsorption capacity (mmol·g–1) and adsorption strength (L·mmol–1), respectively.
where, β is the coefficient of free sorption energy (mol2·kJ-2) and E is the apparent energy of adsorption (kJ·mol–1).
The relative surface adsorption sites and atom ratios were obtained using a high-resolution transmission electron microscopy-energy dispersive X-ray spectroscopy (HRTEM-EDS) system JEOL-2010 (JEOL, Tokyo, Japan) (Liu et al. Reference Liu, Xie, Su, Du, Zhang and Cao2014b). The Parstat 4000 electrochemical workstation with a three-electrode system (Princeton, New Jersey, USA) was used for cyclic voltammetry testing (He et al. Reference He, Zong, Dong, Yang, Ke and Liu2017). FTO (SnO2:F) conductive glass was used as a working electrode, with an electrode reaction area of 15 mm × 25 mm.
Results and Discussion
Structural Characterization of MFe2O4-montmorillonite
In the XRD pattern of MFe2O4-montmorillonite (Fig. 1), the diffraction peaks at 6.5°2θ and 19.8°2θ were assigned to the (001), (101) faces, respectively, consistent with montmorillonite-15A (JCPDS No: 29-1498) (Chang et al. Reference Chang, Ma, Ma, Zhang, Qiao and Hu2015). Besides, the characteristic reflections at 18.2, 30.0, 35.4, 43.0, 53.4, 56.9, and 62.5°2θ, corresponding to the crystal surfaces of (111), (220), (311), (400), (422), (511), and (440), respectively, demonstrate the existence of the MFe2O4 pure phase (Fe3O4, JCPDS-No: 19-0629; MnFe2O4, JCPDS-No: 10-0319; CoFe2O4, JCPDS-No: 22-1086; ZnFe2O4, JCPDS-No: 89-1010 and NiFe2O4, JCPDS-No: 87-2338) (Wang et al. Reference Wang, Li, Wang, Zhao and Jiang2012a; Hu et al. Reference Hu, Gao and Yang2008). Moreover, other characteristic peaks were not observed, suggesting that as-synthesized MFe2O4-montmorillonite with good crystallinity was prepared successfully.

Fig. 1 XRD patterns of MFe2O4-montmorillonite
To further demonstrate the bond structures of the MFe2O4-montmorillonite, the bonding patterns data were analyzed with FTIR and Raman spectroscopies. The double absorption peaks near 594 cm–1 are assigned to the stretching band of Fe–O in MFe2O4 octahedra and tetrahedra (Fig. 2a), and the other typical bands of MFe2O4 were also observed at 1380 cm–1 and 2920 cm–1. Moreover, the frequency of 1090 cm–1 represents the stretching bands of Si–O in montmorillonite, and 3630 cm–1 represents the octahedral O–H stretching bands. The other absorption peaks at 3430 cm–1 and 1640 cm–1 are assigned to the O-H stretch vibrations and the H-O-H bending vibration of H2O, respectively. In the Raman patterns (Fig. 2b), the signature peaks of Eg and A1g bands in MFe2O4 appeared at 303 cm–1 and 667 cm–1, respectively. In addition, the Raman shift around 1600 cm–1 was observed, and the weak peak was predicted to be attributed to the long-range electronic transition bonds of AlO–OFe(M), which suggests the formation of interface oxygen vacancies that could capture the -OH groups from water molecules (Li et al. Reference Li, Yi, Tang, Liu, Wang and Cui2016). From the data above, the as-synthesized samples consisted of MFe2O4 and montmorillonite.

Fig. 2 (a) FTIR patterns and (b) Raman spectra of MFe2O4-montmorillonite
SEM and TEM examinations of MFe2O4-montmorillonite were performed to explore the morphology and interface structure properties of samples. A typical SEM image of MFe2O4-montmorillonite revealed that the magnetic Fe3O4 nanoparticles grew on montmorillonite layer surfaces with unavoidable agglomeration (Fig. 3a). Fe3O4 (–34690.42 eV) had a lower crystal energy than MFe2O4 (–33000.22 eV, –35432.89 eV, –38601.21 eV, –36106.85 eV, M = Mn, Zn, Ni, or Co) (Bian et al. Reference Bian, Li, Li, Nie, Dong, Dong, Bian, Li and Li2017). Hence, the inhibited agglomeration could be observed clearly, whereas the average particle size increased from 60 to ~200 nm. In the TEM images, the agglomeration of nanoparticles was observed more intuitively, and the MFe2O4 grew primarily on the edge sites of montmorillonite (Fig. 4a). The relative lattice distances (d) and crystal faces were measured based on HRTEM images (Fig. 4b), as shown in Table 1. Compared with the reported values, the difference of MFe2O4-montmorillonite faces ranged from 0.01 to 0.03 nm. For the MFe2O4 crystal, the typical sites for Fe2+ in Fe3O4 were occupied by M 2+ ions. Due to the differences in possible atomic radius of M 2+ ions (0.153 nm to ~0.167 nm) and Fe2+ (0.179 nm) (Sun et al. Reference Sun, Zeng, Robinson, Raoux, Rice, Wang and Li2004; Gao et al. Reference Gao, Liu, Zhu, Wang and Xie2016), Fe-O tetrahedral shrinkage affected the lattice of Fe2O3 octahedra nearby, decreasing the lattice distances of (111)/(311) by 0.01 nm and increasing that of (220) by 0.01 nm. The surface energy (1.06 to ~1.18 J·m-2) of the (111) face in MFe2O4 was less than that of a high-index (311) face (Yu et al. Reference Yu, Huo, Li, Wang and Jiao2012), thus the MFe2O4(111) combined preferentially with the exposed Al2O3(100) face of the montmorillonite edge sites (Hong et al. Reference Hong, Kim, Fang, Hong and Chiang2017). In addition, the data reflected the good matching with MFe2O4(111) faces and exposed Al2O3(100). Furthermore, HRTEM-EDS analysis was conducted to analyze the element distribution. Fe and O on the montmorillonite surfaces proved the uniform distribution of MFe2O4 (Fig. 3b). In addition, the results revealed that the atomic ratio of Si:Al reached 18:1, indicating that the Al3+ in the montmorillonite crystal lattice was often replaced by some low-valence cations in solution (e.g. Fe2+, Zn2+, Mg2+) (Brigatti et al. Reference Brigatti, Galán, Theng, Bergaya, Theng and Lagaly2013). However, the exposed Al-O octahedron on the edge of montmorillonite still exhibited high activity, and it could provide active electrons for the adsorption reaction (Hennig et al. Reference Hennig, Reich, Dahn and Scheidegger2002).

Fig. 3 (a) SEM images of MFe2O4-montmorillonite and (b) HRTEM-EDS images of MnFe2O4-montmorillonite

Fig. 4 (a) HRTEM and (b) TEM images of MFe2O4-montmorillonite
Table 1 The lattice distance (d) data and crystal faces of MFe2O4-montmorillonite

F represents MFe2O4 and M represents montmorillonite.
Expermenta-d: d data and crystal planes reported from HRTEM (Phumying et al. Reference Phumying, Labuayai, Swatsitang, Amornkitbamrung and Maensiri2013; Liu et al. Reference Liu, Fang, Jing, Li, Li and Chen2014a; Gao et al. Reference Gao, Liu, Zhu, Wang and Xie2016; Yan et al. Reference Yan, Li, Yu, Shan, Du and Liu2016).
Present work: HRTEM results.
The surface composition of MFe2O4-montmorillonite was further demonstrated using XPS. The characteristic peaks appearing at 72.7 eV, 99.5 eV, and 150.8 eV are assigned to Al 2p3 (Al3+), Si 2p3, and Si 2s (Si4+) in the montmorillonite carrier, and the 725.5 eV corresponded to Fe 2p3/2 (Fe3+) ions coexisting in the MFe2O4 (Fig. 5). Moreover, an interesting peak of 712 eV, indicating the presence of Fe 2p1/2 (Fe2+), was observed in each MFe2O4-montmorillonite. This abnormal phenomenon could occur as the active electrons transferred to MFe2O4, as mentioned previously, resulting in some Fe3+ ions being reduced to Fe2+. The other characteristic peaks of Mn 2p (Mn2+), Co 2p (Co2+), Ni 2p (Ni2+), and Zn 2p (Zn2+) were demonstrated clearly with binding energies at 641 eV, 784eV, 855 eV, and 1022 eV, respectively. The detailed peak-fitting data are shown in Supplementary Fig. S2. The presence of M 2+ is thus strong evidence for homogeneous bonding in MFe2O4, and the samples consisted of montmorillonite with MFe2O4.

Fig. 5 XPS spectra of MFe2O4-montmorillonite
Electron-Transfer Mechanism of MFe2O4-montmorillonite
In general, adsorption behavior was affected by the surface potential of the adsorbent. In the montmorillonite, the interlayer potential showed a permanent negative charge by isomorphic substitution (Zuo et al. Reference Zuo, Gao, Yang, Zhang, Chen, Li, Shi and Wu2017); the potentials of MFe2O4 were reported as 17.5 to ~20 mV (M = Mn, Fe, Zn) and –15 to ~–30 mV (M = Co, Ni) at pH=5–6 (Chen et al. Reference Chen, Wen, Kong, Tian, Ding and Xiong2014; Jiang et al. Reference Jiang, Sun, Xu, Lu and Shi2016; Liu et al. Reference Liu, Yang and Zhang2016; Tu et al. Reference Tu, Chan, Tu, Wang, You and Chang2016; Deng et al. Reference Deng, Chen, Lu, Ma, Feng, Gao and Li2017). Moreover, the zeta potential of MFe2O4-montmorillonite was measured as –6.5 to ~26 mV at pH 5.5 (Fig. 6a), and the potential decreased with the rise in pH because increasing OH- was adsorbed to the edge surfaces of the montmorillonite (Min and Lee Reference Min and Lee2010). Thus, the MFe2O4 on the surface of montmorillonite was vital for the surface potential of the adsorbent (Fig. 6b). At the interfaces, M 2+ in MFe2O4, an electron acceptor, could provide an oxygen vacancy and lead to active electrons at the montmorillonite edge sites being transferred to compensate the electron holes (Bian et al. Reference Bian, Song, Dong, Duan, Xu and Li2015). Thus, MFe2O4-montmorillonite exhibited greater positive surface potential than Fe3O4-montmorillonite. In addition, the zeta potential was used to describe the dispersion stability of the adsorbent in aqueous solution. The potential of MFe2O4-montmorillonite (M = Co, Ni, Zn) was ascertained to be 8.9 to ~41 mV, which indicates that the adsorbent systems were stable. The pH of zero potential for Fe3O4-montmorillonite was ~5.5, suggesting the interactions between metal ions and adsorbent were easier to enhance at pH < 5.5. Thus, the adsorption experiments were carried out at pH 5.5.

Fig. 6 (a) Zeta potential curves and (b) surfaces/interfaces/interlayer charge distribution of MFe2O4-montmorillonite
The UV-Vis diffuse reflectance spectra were obtained to investigate further the mechanism of electron transfer at the MFe2O4-montmorillonite surface and interface. Also, the band gaps were estimated by the photon energy equation:
where h denotes Planck’s constant; k is a constant (1.6×10–19 J·eV–1); C/λ is wave frequency based on the tangent line; Eg is band gap energy (eV).
The band gap of montmorillonite was calculated as 2.5 to ~3.06 eV and MFe2O4 was ~1.62 eV (Fig. 7a). Due to the particle confinement effect as reported by Fatimah et al. (Reference Fatimah, Wang and Wulandari2011), the band gap energy of MFe2O4-montmorillonite decreased, and the peak shifted to a higher wavelength compared with Fe3O4-montmorillonite. This result implied that the addition of MFe2O4 led to an increase in particle size. It is noteworthy that a small band gap (0.78 to ~0.82 eV) appeared near 1900 nm. Electron holes could be formed due to the presence of M 2+ mediators to receive and transmit electrons which were provided by the electron donor (Fig. 7b) (Rekhila et al. Reference Rekhila, Trari and Bessekhouad2017), this suggested that the interfaces of MFe2O4-montmorillonite were the main region for electron transfer.

Fig. 7 (a) DRS curves of MFe2O4-montmorillonite and (b) the relative electron transfer reaction
The interface properties of MFe2O4-montmorillonite were supported by THz-TDS spectra. The peak widths of equipment-emitted pulses were <0.65 ps, and the amplitudes were nearly 100 to ~150 V (Fig. 8a). With the strong absorption of terahertz rays, light could pass easily through the montmorillonite edge sites without changing velocity (Li et al., 2017). In the refraction index information, the two absorption peaks were near 1.75 THz, which are assigned to the photon adsorption of the O-Fe (2p1/2) octahedron and O-Fe (2p3/2) tetrahedron in the MFe2O4 (Fig. 8b). The refraction peaks at ~2.15 THz reflected the MFe2O4(111)-montmorillonite(100) interfaces, because poorly active electrons at the interfaces were triggered and detected easily. In addition, note that MFe2O4-montmorillonite showed a smaller refractive index peak and strong absorption at 1.95 to ~2.15 THz in the absorption spectrum (Fig. 8c). This abnormal phenomenon was predicted as the electronic transition within the interfacial bonding of AlO-OFe(M) and SiO-OFe(M) between the montmorillonite and MFe2O4, associated with the synergistic hybridized orbitals effect of O-O sp2, etc. The tangent data were calculated to explain better the electronic mechanism of the interfaces, according to the frequency difference (Fig. 8d). The tangent data revealed that the concentration of surface oxygen vacancies of MFe2O4(111) increased by the paired electrons of M 2+. Therefore, the active electrons of MFe2O4(111)-montmorillonite(100) interfaces were of significance to the adsorption of UO2 2+-X n+ from solution.

Fig. 8 (a) Time dependence, (b) refraction index, (c) absorption, and (d) frequency difference (FD) power of THz signal spectra of the MFe2O4-montmorillonite interfaces, where time-resolved spectra of semicoke were obtained via THz-TDS. The time resolution was on a picosecond scale
Competitive Adsorption of Toxic Trace Metal Ions Using MFe2O4-montmorillonite
To evaluate the adsorption capacity of the adsorbent, data were obtained through ICP measurements. The results revealed that the equilibrium adsorption capacity increased with the increase in initial metal ion concentration (Fig. 9). The total adsorption capacities of UO2 2+ and metal ions were 322 to ~396 mg·g–1 in the initial ionic concentration of 60 mg·L–1 (Supplementary Table S1). Subsequently, Langmuir, Freundlich, and Dubinin-Radushkevich models were adopted to describe the adsorption behavior (Table 2). The Langmuir linear correlation coefficients (R2) were calculated as 0.913 to ~0.999, larger than those of Freundlich 0.774 to ~0.999. The Langmuir isotherm fitted the experimental data better. The results revealed that Rb+, Sr2+, Mn2+, Ni2+, Zn2+, Cd2+, and Cr3+ ions were primarily at the adsorbent surfaces as a result of monolayer physical adsorption. However, the values of R2 for UO2 2+ were approximately 0.897 to ~0.989 (Freundlich) and 0.739 to ~0.977 (Langmuir). The exception showed that the adsorption of UO2 2+ was more consistent with the Freundlich isotherm, without that of Fe3O4-montmorillonite. The Freundlich constants (1/n) were associated with the adsorption intensity, and the constants of 1/n of X n+ were calculated as 0.5 to ~3.2 (0.5<1/n<1), which indicated that the adsorption of Rb+, Sr2+, Mn2+, Ni2+, Zn2+, and Cd2+ ions would be favorable. The constants of 1/n of X n+ UO2 2+ adsorption were 0.09 to ~0.37, within the range of 0.1 < 1/n < 0.5, which suggests that the UO2 2+ was more significantly adsorbed on the adsorbent. In addition, the linear correlation coefficients (R2) of the D–R isotherm for X n+ and UO2 2+ were determined as 0.913 to ~0.997. This indicated that X n+ and UO2 2+ ions were also adsorbed well in the interlayers of montmorillonite. On the whole, the difference in adsorption-fitting results was associated with the competitive adsorption between UO2 2+ and X n+.

Fig. 9 The equilibrium adsorption capacity data of MFe2O4-montmorillonite from heavy metal mixed solution (ppm, parts per million, indicates the concentration of the solute)
Table 2 Isotherm parameters for the uranyl-toxic metal MFe2O4-montmorillonite

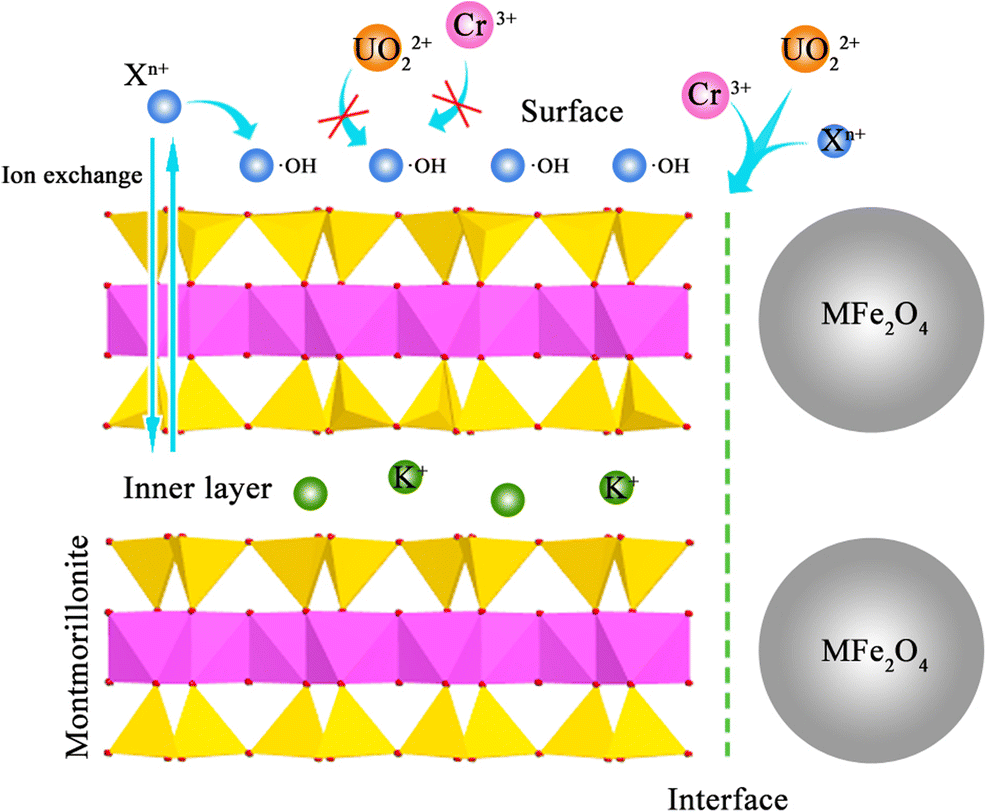
The adsorption mechanism was considered to be by ligand exchange of hydroxyl groups and an adsorption reaction caused by the transition of electrons in the long-range AlO–OFe(M) bonds, creating surface hydroxyl radical layers in aqueous solution and changing the adsorption behavior from monolayer to multilayer. In the adsorption behaviors, involving successive mono electronic transition, UO2 2+-X n+ adsorption could be assumed to be the direct electron transfer for increasing •OH and the complexation of •OH with UO2 2+ or X n+ (Karamanis Reference Karamanis1997). According to previous reports (Xu et al. Reference Xu, Zhang, Wang, Xu, Ding and Li2013), the UO2 2+ and X n+ ions underwent competitive adsorption on the surfaces of montmorillonite, where the adsorption reaction of surface •OH with positively charged X n+ could form an ion monolayer to block UO2 2+ from being adsorbed. The adsorption results implied that the adsorption positions of UO2 2+ and X n+ were not the same as those on the surfaces of MFe2O4-montmorillonite, and the adsorption of UO2 2+ occurred primarily at interfaces. Take ZnFe2O4-montmorillonite as an example. The adsorption of X n+ overall fitted the Langmuir and Freundlich models (Fig. 10a), whereas the maximum adsorption capacity of Freundlich was much lower than the actual adsorption capacity, which revealed that the X n+ were adsorbed primarily by monolayer physical adsorption on the adsorbent surfaces. However, the Freundlich model described the adsorption of Cr3+ and UO2 2+ better than the Langmuir model (Fig. 10b), and the saturated UO2 2+ adsorption capacity of different models differed significantly. Thus, the adsorption positions of UO2 2+ were primarily at ZnFe2O4-montmorillonite interfaces.

Fig. 10 (a) The fitting curves of Sr2+ and (b) UO2 2+, Cr3+ to Freundlich, Langmuir, and Dubinin-Radushkevich models at ZnFe2O4-montmorillonite surfaces
The maximum adsorption capacity was calculated from the adsorption models. The results revealed that MFe2O4-montmorillonite had a better adsorption capacity of metal ions using Langmuir and D-R equation fitting (Fig. 11a-b). However, the adsorption capacities of UO2 2+ and Cr3+ were much greater than that of other X n+ ions using the Freundlich model (Figure 11c). The data demonstrated the highly selective adsorption behavior of UO2 2+ and Cr3+ ions in MFe2O4-montmorillonite interfaces (Fig. 11d); the illustration of the adsorption mechanism has been mentioned. The interface adsorption capacity of UO2 2+ was 25.1 mg·g–1 and that of Cr3+, 60.2 mg·g–1. Additionally, the maximum adsorption capacity of Cr3+ from the Langmuir isotherm was 163.8 mg·g–1. Compared with previous reports, the adsorption capacities of UO2 2+ and Cr3+ were just 5 to ~33.7 mg·g–1 using montmorillonite and other modified Fe3O4, and the MFe2O4-montmorillonite exhibited a large adsorption capacity of UO2 2+, as listed in Table 3. Thus, the medium (M 2+) and material modifications of Fe3O4 were proven as one of the effective ways to enhance adsorption capacity. Though the adsorption reaction was known to be a spontaneous and endothermic process, the maximum adsorption capacities of X n+ ions using Langmuir (39.7 to ~449.4 mg·g–1) were greater than the capacities using the Dubinin–Radushkevich equation (15.1 to ~95.4 mg·g–1). Thus, the weak electrostatic attraction and the hydroxyl adsorption reaction between MFe2O4-montmorillonite and X n+ ions remained the major process for metal-ion adsorption, compared with adsorption by interlayer ion exchange.

Fig. 11 Maximum adsorption capacities of uranyl and toxic metal ions at MFe2O4-montmorillonite surfaces/interfaces/interlayer. (a) Langmuir, (b) Dubinin-Radushkevich, (c) Freundlich models, and (d) Illustration of the selective adsorption mechanism
Table 3 Comparison of UO2 2+ and Cr3+ ion-adsorption performance among various adsorbents

HRTEM-EDS images were used to verify the presence of X n+ and UO2 2+ ions after adsorption. The elemental distribution of Fe3O4-montmorillonite was observed, and the UO2 2+/X n+ ions were found to have been adsorbed uniformly in the sample (Fig. 12a). Ignoring dissociation of the X n+ ions, the elemental distribution of MFe2O4-montmorillonite after desorption showed a strong response to the existence of UO2 2+ and Cr3+ (Fig. 12b). Thus, the collaborative adsorptions of UO2 2+ and Cr3+ should be noted. The UO2 2+ preferentially compensated electron holes and adsorbed at the interfaces compared with Cr3+ metal ions, reported as Cr3+4s1 electron orbital, which is easier to protonate than the U 5f36d1 orbital (De et al. Reference De Decker, Folens, De Clercq, Meledina, Van Tendeloo, Du Laing and Van Der Voort2017). Consequently, the selective adsorption of UO2 2+ was enhanced through the active electron/hole regulation by the M 2+ ionic medium modification, and the adsorption of UO2 2+ was treated as favorable.

Fig. 12 (a) HRTEM-EDS of uranyl-toxic metal ions in Fe3O4-montmorillonite and (b) HRTEM-EDS of uranyl-toxic metal ions in MFe2O4-montmorillonite after desorption, where “at.%” means the atom ratio
The charge transition of interaction between UO2 2+ and X n+ was characterized using cyclic voltammetry. The appearance of the reduction peak revealed that UO2 2+ ions could be reduced to UO2 + by gaining electrons when the potential was scanned from positive to negative (Fig. 13a). However, only a portion of UO2 + oxidized back to UO2 2+ compared with the integral area of the redox reaction, suggesting that UO2 2+ was oxidized heterogeneously to UO2 + (Singer et al. Reference Singer, Chatman, Ilton, Rosso, Banfield and Waychunas2012) and the adsorption of UO2 2+ at the interfaces was stable. The X n+ ions did not undergo redox reaction throughout the cycle, because the exchange of active electrons took place primarily in the interface layer. For instance, the oxidation-reduction peaks of Sr2+ ions did not appear (Fig. 13b). However, the integral reduction area of Cr3+ ions was expanded, suggesting that some of the Cr3+ was reduced to Cr2+. In addition, the coupling of electrons with Cr2+-Cr3+ co-orbitals on the interfaces led to inhibition of the oxidation. The total peaks were obtained through electric signals covering (Fig. 13c), and the total oxidation signal was weakened. This situation predicted that active electrons transferred from Cr3+ to UO2 + on the interfaces and formed UO2 2+-UO2 +-Cr3+-Cr2+ redox reactions (Fig. 13d). Hence, the selective adsorption of UO2 2+ in the MFe2O4-montmorillonite interfaces was not only associated with electrostatic interaction but was also affected by complexation of Cr3+ ions.

Fig. 13 Cyclic voltammograms of FTO glass electrode in 60 ppm solute concentrations. (a) UO2 2+, (b) Cr3+-Sr2+, (c) the mixed uranyl-toxic metal ion solution (pH 5.5), and (d) the charge transition mechanism at MFe2O4(311)/(111)-montmorillonite(100) interfaces. Therein, the scan rate was 25 mV∙s–1, “O” and “R” represent the oxidation and reduction peaks, respectively (in parts a–c, the horizontal axis, E (V vs. SCE) represents the scanning potential and the vertical axis, (J (mA∙cm–3)), the current density (i.e. the ratio of the current to the interface area of the electrode at one potential)
Conclusion
The MFe2O4-montmorillonite adsorbent was synthesized for removal of UO2 2+ from solutions coexisting with toxic trace-metal ions. The results of structural analysis indicated that MFe2O4 grew primarily on the edge site of montmorillonite, and the intervention of M 2+ ions effectively inhibited the agglomeration of MFe2O4. Moreover, the interfacial activity of MFe2O4-montmorillonite increased through the electronic transition of Al(Si)-O-O-M bonding, and the interface effect could promote the selective removal of UO2 2+ in an adsorbent. The adsorption results revealed that the X n+ (Rb+, Sr2+, Mn2+, Ni2+, Zn2+, Cd2+, and Cr3+) ions were primarily on the MFe2O4-montmorillonite surfaces, which is consistent with monolayer physical adsorption behavior. As a result of the monolayer of the •OH complexes with X n+, UO2 2+ ions were absorbed at the interfaces. Further study suggested that UO2 2+ and Cr3+ ions had redox reactions and underwent synergistic adsorption on the interfaces of MFe2O4-montmorillonite. The interface maximum adsorption capacities of UO2 2+ and Cr3+ were 25.1 mg·g–1 and 60.2 mg·g–1, respectively.
All results were consistent with the initial hypothesis, supporting the conclusion that the medium (M 2+) of Fe3O4 and modifications using a montmorillonite matrix were effective ways to enhance the adsorption capacity and the adsorption selectivity of UO2 2+. Thus, MFe2O4-montmorillonites were shown to be potential adsorbents for uraniferous heavy metal wastewater treatment. Future investigation will focus on the applications of adsorbents in uranium mine wastewater, etc.
Electronic supplementary material
The online version of this article (https://doi.org/10.1007/s42860-019-00028-x) contains supplementary material, which is available to authorized users.
Acknowledgments
The authors acknowledge funding from the National Natural Science Foundation of China (41872039 and 41831285), the One-Thousand Talents Scheme in Sichuan Province, Sichuan Science and Technology Program (2018JY0462), and Longshan Fund of Southwest University of Science and Technology (17QR004).