Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Madejová, Jana
Pálková, Helena
Pentrák, Martin
and
Komadel, Peter
2009.
Near-infrared spectroscopic analysis of acid-treated organo-clays.
Clays and Clay Minerals,
Vol. 57,
Issue. 3,
p.
392.
Sánchez del Río, Manuel
Boccaleri, Enrico
Milanesio, Marco
Croce, Gianluca
van Beek, Wouter
Tsiantos, Constantinos
Chyssikos, Georgios D.
Gionis, Vassilis
Kacandes, George H.
Suárez, Mercedes
and
García-Romero, Emilia
2009.
A combined synchrotron powder diffraction and vibrational study of the thermal treatment of palygorskite–indigo to produce Maya blue.
Journal of Materials Science,
Vol. 44,
Issue. 20,
p.
5524.
Madejová, Jana
Pentrák, Martin
Pálková, Helena
and
Komadel, Peter
2009.
Near-infrared spectroscopy: A powerful tool in studies of acid-treated clay minerals.
Vibrational Spectroscopy,
Vol. 49,
Issue. 2,
p.
211.
Garciá-Romero, E.
and
Suárez, M.
2010.
On the Chemical Composition of Sepiolite and Palygorskite.
Clays and Clay Minerals,
Vol. 58,
Issue. 1,
p.
1.
Mora, Manuel
Isabel López, M.
Ángeles Carmona, M.
Jiménez-Sanchidrián, C.
and
Rafael Ruiz, J.
2010.
Study of the thermal decomposition of a sepiolite by mid- and near-infrared spectroscopies.
Polyhedron,
Vol. 29,
Issue. 16,
p.
3046.
Madejová, Jana
Pálková, Helena
and
Komadel, Peter
2010.
Spectroscopic Properties of Inorganic and Organometallic Compounds.
p.
22.
Madejová, Jana
Balan, Etienne
and
Petit, Sabine
2010.
Advances in the characterization of industrial minerals.
p.
171.
Anbalagan, G.
Sivakumar, G.
Prabakaran, A.R.
and
Gunasekaran, S.
2010.
Spectroscopic characterization of natural chrysotile.
Vibrational Spectroscopy,
Vol. 52,
Issue. 2,
p.
122.
Goldberg, Sabine
and
Suarez, Donald L.
2011.
Distinguishing Boron Desorption from Mineral Dissolution in Arid‐Zone Soils.
Soil Science Society of America Journal,
Vol. 75,
Issue. 4,
p.
1347.
Giustetto, R.
and
Compagnoni, R.
2011.
An unusual occurrence of palygorskite from Montestrutto, Sesia-Lanzo zone, internal Western Alps (Italy).
Clay Minerals,
Vol. 46,
Issue. 3,
p.
371.
Chen, Hao
Zhao, Jie
Zhong, Aiguo
and
Jin, Yanxian
2011.
Removal capacity and adsorption mechanism of heat-treated palygorskite clay for methylene blue.
Chemical Engineering Journal,
Vol. 174,
Issue. 1,
p.
143.
Sánchez del Río, Manuel
Doménech, Antonio
Doménech-Carbó, María Teresa
Vázquez de Agredos Pascual, María Luisa
Suárez, Mercedes
and
García-Romero, Emilia
2011.
Developments in Palygorskite-Sepiolite Research.
Vol. 3,
Issue. ,
p.
453.
Madejová, Jana
Jankovič, Ľuboš
Pentrák, Martin
and
Komadel, Peter
2011.
Benefits of near-infrared spectroscopy for characterization of selected organo-montmorillonites.
Vibrational Spectroscopy,
Suárez, Mercedes
and
García-Romero, Emilia
2011.
Developments in Palygorskite-Sepiolite Research.
Vol. 3,
Issue. ,
p.
33.
Tsiantos, Constantinos
Tsampodimou, Maria
Kacandes, George H.
Sánchez del Río, Manuel
Gionis, Vassilis
and
Chryssikos, Georgios D.
2012.
Vibrational investigation of indigo–palygorskite association(s) in synthetic Maya blue.
Journal of Materials Science,
Vol. 47,
Issue. 7,
p.
3415.
Bukas, Vanessa Jane
Tsampodimou, Maria
Gionis, Vassilis
and
Chryssikos, Georgios D.
2013.
Synchronous ATR infrared and NIR-spectroscopy investigation of sepiolite upon drying.
Vibrational Spectroscopy,
Vol. 68,
Issue. ,
p.
51.
Rhouta, Benaissa
Zatile, Ezzouhra
Bouna, Lahcen
Lakbita, Omar
Maury, Francis
Daoudi, Lahcen
Lafont, Marie Christine
Amjoud, M’Barek
Senocq, François
Jada, Amane
and
Aït Aghzzaf, Ahmed
2013.
Comprehensive physicochemical study of dioctahedral palygorskite-rich clay from Marrakech High Atlas (Morocco).
Physics and Chemistry of Minerals,
Vol. 40,
Issue. 5,
p.
411.
Petit, S.
and
Madejova, J.
2013.
Handbook of Clay Science.
Vol. 5,
Issue. ,
p.
213.
Suárez, Mercedes
and
García-Romero, Emilia
2013.
Sepiolite-Palygorskite: A Continuous Polysomatic Series.
Clays and Clay Minerals,
Vol. 61,
Issue. 5,
p.
461.
Skoubris, Evangelos N.
Chryssikos, Georgios D.
Christidis, George E.
and
Gionis, Vassilis
2013.
Structural Characterization of Reduced-Charge Montmorillonites. Evidence Based on FTIR Spectroscopy, Thermal Behavior, and Layer-Charge Systematics.
Clays and Clay Minerals,
Vol. 61,
Issue. 2,
p.
83.
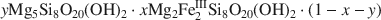 Mg2Al2Si8O20(OH)2. Included in the study were PFl-1 and several commercial palygorskites. Our analysis of 2nd derivative NIR spectra shows that the dioctahedral composition is adequately described by three sharp overtone bands representing AlAlOH, AlFeIIIOH and FeIIIFeIIIOH in M2 dioctahedral sites, and that the summed intensity of these bands is proportional to the amount of dioctahedral component present (1−y). The samples show large variations in the degree of dioctahedral FeIII-for-Al substitution with FeIII occupying up to 70% of the dioctahedral M2 sites. Ternary analysis shows that the distribution of dioctahedral Al and FeIII is not random, but displays a tendency towards homoionic pairing. An overtone band at 7214 cm−1 and several combination bands are indicative of a trioctahedral Mg3OH component (y), and their appearance correlates with a distinct palygorskite signature in thermogravimetric analysis. Nevertheless, these bands cannot be used reliably for the quantification of a trioctahedral palygorskite component due to their close similarity to those of sepiolite. To circumvent this problem, we have evaluated y indirectly by calculating the difference between 1−y and the total concentration of palygorskite determined by the normalized intensity of the d110 XRD peak of palygorskite at 10.4 Å. Using this methodology, we have found that the samples conform to a trioctahedral limit of y ≈ 0.55, although within this limit they display large variations in octahedral character. Finally, we extend the above methodology to PLS chemometrics and show how NIR can be used to determine palygorskite content routinely in multimineralic geological samples.
Mg2Al2Si8O20(OH)2. Included in the study were PFl-1 and several commercial palygorskites. Our analysis of 2nd derivative NIR spectra shows that the dioctahedral composition is adequately described by three sharp overtone bands representing AlAlOH, AlFeIIIOH and FeIIIFeIIIOH in M2 dioctahedral sites, and that the summed intensity of these bands is proportional to the amount of dioctahedral component present (1−y). The samples show large variations in the degree of dioctahedral FeIII-for-Al substitution with FeIII occupying up to 70% of the dioctahedral M2 sites. Ternary analysis shows that the distribution of dioctahedral Al and FeIII is not random, but displays a tendency towards homoionic pairing. An overtone band at 7214 cm−1 and several combination bands are indicative of a trioctahedral Mg3OH component (y), and their appearance correlates with a distinct palygorskite signature in thermogravimetric analysis. Nevertheless, these bands cannot be used reliably for the quantification of a trioctahedral palygorskite component due to their close similarity to those of sepiolite. To circumvent this problem, we have evaluated y indirectly by calculating the difference between 1−y and the total concentration of palygorskite determined by the normalized intensity of the d110 XRD peak of palygorskite at 10.4 Å. Using this methodology, we have found that the samples conform to a trioctahedral limit of y ≈ 0.55, although within this limit they display large variations in octahedral character. Finally, we extend the above methodology to PLS chemometrics and show how NIR can be used to determine palygorskite content routinely in multimineralic geological samples.