Published online by Cambridge University Press: 01 July 2024
Chlorite and illite are the major clay minerals in silicate assemblages from a rock salt bed in the Vernon Formation (Upper Silurian) at Retsof, New York. Textural features and Br content of the salt indicate precipitation from shallow marine brine with no subsequent postdepositional recrystallization. Sample mounting procedure for electron microprobe analysis involves clay particle dispersion, sedimentation, and transferral to a planar silver print surface. The 001 face of the flake, rather than the conventional polished plane, constitutes the analyzed surface. Microprobe analysis of the chlorite (80 grains from four samples) yields a mean aggregate Mg-rich clinochlore composition of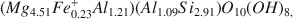
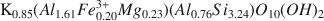
Diagenesis effected improved crystallinity and undoubtedly involved isochemical recrystallization of the bulk silicate assemblage. Metasomatism of the assemblage during diagenesis, however, appears to be negligible.