Published online by Cambridge University Press: 01 January 2024
Because of the anisotropy in bonding, layered hydroxides crystallize with extensive structural disorder due to the incorporation of stacking faults. In contrast, the loss of crystallinity in Br−-ion intercalated layered double hydroxides (LDHs) arises due to the positional disorder of Br− in the interlayer. The structure of the interlayer in other LDHs is poorly understood due to the low X-ray scattering power of the commonly found anions such as Cl− and NO3−\$\end{document}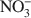 relative to that of the metal hydroxide layers. On heating to 175°C, the Br− ion migrates from positions of lesser site degeneracy to those of greater site degeneracy as dehydration of the interlayer opens up access to positions hitherto occupied by intercalated water molecules. The new (18h) site is situated closer to the proton of the metal hydroxide layer (1.809 Å) compared to the 6c site (2.402 Å). This shows a pre-association of the bromide ion with the proton of the hydroxide layer leading to the release of HBr upon decomposition of the bromide-containing LDHs. The fact that Cl−-containing LDHs also decompose with the evolution of HCl shows that such a redistribution of the atoms in the interlayer is more common than is generally recognized.
relative to that of the metal hydroxide layers. On heating to 175°C, the Br− ion migrates from positions of lesser site degeneracy to those of greater site degeneracy as dehydration of the interlayer opens up access to positions hitherto occupied by intercalated water molecules. The new (18h) site is situated closer to the proton of the metal hydroxide layer (1.809 Å) compared to the 6c site (2.402 Å). This shows a pre-association of the bromide ion with the proton of the hydroxide layer leading to the release of HBr upon decomposition of the bromide-containing LDHs. The fact that Cl−-containing LDHs also decompose with the evolution of HCl shows that such a redistribution of the atoms in the interlayer is more common than is generally recognized.