Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Schampera, B.
and
Dultz, S.
2009.
Determination of diffusive transport in HDPy-montmorillonite by H2O-D2O exchange using in situ ATR-FTIR spectroscopy.
Clay Minerals,
Vol. 44,
Issue. 2,
p.
249.
Ruiz-Hitzky, Eduardo
Aranda, Pilar
Darder, Margarita
and
Ogawa, Makoto
2011.
Hybrid and biohybrid silicate based materials: molecular vs. block-assembling bottom–up processes.
Chem. Soc. Rev.,
Vol. 40,
Issue. 2,
p.
801.
Dultz, Stefan
An, Jong-Hyok
and
Riebe, Beate
2012.
Organic cation exchanged montmorillonite and vermiculite as adsorbents for Cr(VI): Effect of layer charge on adsorption properties.
Applied Clay Science,
Vol. 67-68,
Issue. ,
p.
125.
Kumar, A. Santhana Krishna
Kalidhasan, S.
Rajesh, Vidya
and
Rajesh, N.
2012.
Application of Cellulose-Clay Composite Biosorbent toward the Effective Adsorption and Removal of Chromium from Industrial Wastewater.
Industrial & Engineering Chemistry Research,
Vol. 51,
Issue. 1,
p.
58.
Darder, Margarita
Aranda, Pilar
and
Ruiz-Hitzky, Eduardo
2012.
Environmental Silicate Nano-Biocomposites.
p.
365.
Cho, Dong-Wan
Jeon, Byong-Hun
Chon, Chul-Min
Kim, Yongje
Schwartz, Franklin W.
Lee, Eung-Seok
and
Song, Hocheol
2012.
A novel chitosan/clay/magnetite composite for adsorption of Cu(II) and As(V).
Chemical Engineering Journal,
Vol. 200-202,
Issue. ,
p.
654.
Anjum, Ansar
Seth, Chand K.
and
Datta, Monika
2013.
Removal of As3+ Using Chitosan–Montmorillonite Composite: Sorptive Equilibrium and Kinetics.
Adsorption Science & Technology,
Vol. 31,
Issue. 4,
p.
303.
Wainipee, Wimolporn
Cuadros, Javier
Sephton, Mark A.
Unsworth, Catherine
Gill, Martin G.
Strekopytov, Stanislav
and
Weiss, Dominik J.
2013.
The effects of oil on As(V) adsorption on illite, kaolinite, montmorillonite and chlorite.
Geochimica et Cosmochimica Acta,
Vol. 121,
Issue. ,
p.
487.
Benhamou, A.
Basly, J.P.
Baudu, M.
Derriche, Z.
and
Hamacha, R.
2013.
Amino-functionalized MCM-41 and MCM-48 for the removal of chromate and arsenate.
Journal of Colloid and Interface Science,
Vol. 404,
Issue. ,
p.
135.
Shinde, Rakesh N.
Pandey, A.K.
Acharya, R.
Guin, R.
Das, S.K.
Rajurkar, N.S.
and
Pujari, P.K.
2013.
Chitosan-transition metal ions complexes for selective arsenic(V) preconcentration.
Water Research,
Vol. 47,
Issue. 10,
p.
3497.
Wilson, Lee D.
Pratt, Dawn Y.
and
Kozinski, Janusz A.
2013.
Preparation and sorption studies of β-cyclodextrin–chitosan–glutaraldehyde terpolymers.
Journal of Colloid and Interface Science,
Vol. 393,
Issue. ,
p.
271.
Koriche, Yamina
Darder, Margarita
Aranda, Pilar
Semsari, Saida
and
Ruiz-Hitzky, Eduardo
2014.
Bionanocomposites based on layered silicates and cationic starch as eco-friendly adsorbents for hexavalent chromium removal.
Dalton Trans.,
Vol. 43,
Issue. 27,
p.
10512.
Wang, Xiaohuan
Yang, Li
Zhang, Junping
Wang, Chuanyi
and
Li, Qiuye
2014.
Preparation and characterization of chitosan–poly(vinyl alcohol)/bentonite nanocomposites for adsorption of Hg(II) ions.
Chemical Engineering Journal,
Vol. 251,
Issue. ,
p.
404.
Szala, Barbara
Bajda, Tomasz
and
Jeleń, Anna
2015.
Removal of chromium(VI) from aqueous solutions using zeolites modified with HDTMA and ODTMA surfactants.
Clay Minerals,
Vol. 50,
Issue. 1,
p.
103.
Prasanthi, Mokkapati Ramya
Jayasravanthi, Mokkapati
and
Nadh, Ratnakaram Venkata
2016.
Kinetic, thermodynamic and equilibrium studies on removal of hexavalent chromium from aqueous solutions using agro-waste biomaterials, casuarina equisetifolia L. and sorghum bicolor.
Korean Journal of Chemical Engineering,
Vol. 33,
Issue. 8,
p.
2374.
Wang, Xianli
Liu, Yukun
and
Zheng, Jingtang
2016.
Removal of As(III) and As(V) from water by chitosan and chitosan derivatives: a review.
Environmental Science and Pollution Research,
Vol. 23,
Issue. 14,
p.
13789.
Padilla-Ortega, Erika
Darder, Margarita
Aranda, Pilar
Figueredo Gouveia, Rubia
Leyva-Ramos, Roberto
and
Ruiz-Hitzky, Eduardo
2016.
Ultrasound assisted preparation of chitosan–vermiculite bionanocomposite foams for cadmium uptake.
Applied Clay Science,
Vol. 130,
Issue. ,
p.
40.
Varghese, Lina Rose
Das, Devlina
and
Das, Nilanjana
2016.
Application of novel nanobiocomposites for removal of nickel(II) from aqueous environments: Equilibrium, kinetics, thermodynamics and ex-situ studies.
Korean Journal of Chemical Engineering,
Vol. 33,
Issue. 1,
p.
238.
Schampera, B.
Šolc, R.
Tunega, D.
and
Dultz, S.
2016.
Experimental and molecular dynamics study on anion diffusion in organically modified bentonite.
Applied Clay Science,
Vol. 120,
Issue. ,
p.
91.
Quintáns-Fondo, Ana
Ferreira-Coelho, Gustavo
Paradelo-Núñez, Remigio
Nóvoa-Muñoz, Juan Carlos
Arias-Estévez, Manuel
Fernández-Sanjurjo, María José
Álvarez-Rodríguez, Esperanza
and
Núñez-Delgado, Avelino
2016.
As(V)/Cr(VI) pollution control in soils, hemp waste, and other by-products: competitive sorption trials.
Environmental Science and Pollution Research,
Vol. 23,
Issue. 19,
p.
19182.
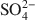 . Chitosan-montmorillonite was prepared by adding an amount of chitosan equivalent to 500% of the cation exchange capacity (CEC) at pH 5 and 75°C. The resulting anion exchange capacity was ∼0.34 molc/kg. The adsorption properties for As(V) and Cr(VI) were determined with the batch technique at pH 3 to 9. Adsorption isotherms were fitted to the Langmuir and Dubinin-Radushkevich equations and judged quantitatively by the correlation coefficient. To describe the competitive adsorption, the selectivity (S) was determined by the ratio of amounts of anions A and B adsorbed (qA/qB) in a binary system. The ionic species adsorbed, i.e. either Cr(VI) or As(V), depended on the pH, as did the degree of protonation of the amine groups, and this played a decisive role in the amount of anions adsorbed. The maximum amount of Cr(VI) adsorbed was 180 mmol/kg at pH 3.5, whereas for As(V) it was 120 mmol/kg at pH 4.0 to 5.0. The adsorption process of Cr(VI) and As(V) fit well to the Langmuir isotherm. By increasing the concentration of the competitive anion, Cl−, in solution, the amount of Cr(VI) and As(V) adsorbed remained almostconstant, whereas SO42−${\rm{SO}}_4^{2 - }$
. Chitosan-montmorillonite was prepared by adding an amount of chitosan equivalent to 500% of the cation exchange capacity (CEC) at pH 5 and 75°C. The resulting anion exchange capacity was ∼0.34 molc/kg. The adsorption properties for As(V) and Cr(VI) were determined with the batch technique at pH 3 to 9. Adsorption isotherms were fitted to the Langmuir and Dubinin-Radushkevich equations and judged quantitatively by the correlation coefficient. To describe the competitive adsorption, the selectivity (S) was determined by the ratio of amounts of anions A and B adsorbed (qA/qB) in a binary system. The ionic species adsorbed, i.e. either Cr(VI) or As(V), depended on the pH, as did the degree of protonation of the amine groups, and this played a decisive role in the amount of anions adsorbed. The maximum amount of Cr(VI) adsorbed was 180 mmol/kg at pH 3.5, whereas for As(V) it was 120 mmol/kg at pH 4.0 to 5.0. The adsorption process of Cr(VI) and As(V) fit well to the Langmuir isotherm. By increasing the concentration of the competitive anion, Cl−, in solution, the amount of Cr(VI) and As(V) adsorbed remained almostconstant, whereas SO42−${\rm{SO}}_4^{2 - }$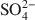 had a more pronounced competitive effect. At concentration ratios of 0.5 and 1 for SO42−${\rm{SO}}_4^{2 - }$
had a more pronounced competitive effect. At concentration ratios of 0.5 and 1 for SO42−${\rm{SO}}_4^{2 - }$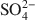 to Cr(VI) and As(V), respectively, the sorption capacity decreased by 10 and 25%, respectively. The sequence of the selectivity was: Cr(VI)>SO42−>As(V)>Cl−${\rm{Cr}}\left( {{\rm{VI}}} \right) > {\rm{SO}}_4^{2 - } > {\rm{As}}\left( {\rm{V}} \right) > {\rm{C}}{{\rm{l}}^ - }$
to Cr(VI) and As(V), respectively, the sorption capacity decreased by 10 and 25%, respectively. The sequence of the selectivity was: Cr(VI)>SO42−>As(V)>Cl−${\rm{Cr}}\left( {{\rm{VI}}} \right) > {\rm{SO}}_4^{2 - } > {\rm{As}}\left( {\rm{V}} \right) > {\rm{C}}{{\rm{l}}^ - }$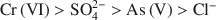 . Chitosan-montmorillonite showed a high selectivity for Cr(VI), which adsorbed chemically. Despite the lower affinity for As(V) and physical adsorption, the adsorption capacity was relatively high.
. Chitosan-montmorillonite showed a high selectivity for Cr(VI), which adsorbed chemically. Despite the lower affinity for As(V) and physical adsorption, the adsorption capacity was relatively high.