Published online by Cambridge University Press: 01 January 2024
Muscovite and K-feldspar (adularia) were dry-ground to — 200/inch particles, which were suspended in water. These suspensions were “titrated” with KCl, and the pH was recorded as a function of KCl concentration and temperature. The results indicate that muscovite and adularia react with water to produce a surface film in which H+ has displaced K+. The “titration” curves show some characteristics attributable to exchange reactions, others apparently related to equilibria among solids of fixed composition. Maximum release of K+ from adularia by reaction with water is much greater than that from mica. The interpretation is made that the first result of reaction of mica and adularia with water is a surface layer that grades from an outer portion that is structurally disrupted to an inner portion that retains the original silicate structure but with H+ substituted for K+. Addition of K+ as KCl to the suspending solution displaces H+ from the disrupted zone, but all H+ originally taken up by the solids was not returned to the solution by concentrations of KCl up to 1.0 M. Experiments were of a few hours duration; work by others has shown that the disrupted zone releases appreciable concentrations of silica and alumina to solution over longer time intervals.
These hydrolysis experiments indicate that at 25°C an H-feldspar or H-mica structure is favored over a K-feldspar or K-mica structure except in solutions in which the ratio of aK+ aH+ exceeds 109–10 or 107–8 respectively. These ratios decrease in the temperature range 25-65°C by a factor of about 100.7. These results, where considered in relation to the observed behavior of feldspar and mica under weathering conditions, indicate that the major energy change for the reactions, $2\,mica + 5{H_2}O = \,3\,kaolin + \,2{K^ + }\, + \,2O{H^ - }$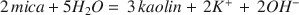 $3K - feldspar + 2{H_2}O = \,K - mica + 6quartz + \,2O{H^ - }$
$3K - feldspar + 2{H_2}O = \,K - mica + 6quartz + \,2O{H^ - }$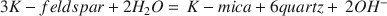 can be considered to result from H+-K+ exchange, and that the energy contribution from other changes is small.
can be considered to result from H+-K+ exchange, and that the energy contribution from other changes is small.