Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Sivamohan, R.
1990.
The problem of recovering very fine particles in mineral processing — A review.
International Journal of Mineral Processing,
Vol. 28,
Issue. 3-4,
p.
247.
Helmy, A. K.
Ferreiro, E. A.
and
de Bussetti, S. G.
1994.
Cation Exchange Capacity and Condition of Zero Charge of Hydroxy-Al Montmorillonite.
Clays and Clay Minerals,
Vol. 42,
Issue. 4,
p.
444.
Gil, A.
and
Montes, M.
1994.
Evolution of the microporous accessibility with the hydrolysis degree and the intercalation solution ageing time conditions in aluminium-pillared clays.
Microporous Materials,
Vol. 3,
Issue. 3,
p.
319.
Gil, A.
Díaz, A.
Montes, M.
and
Acosta, D. R.
1994.
Characterization of the microporosity of pillared clays by nitrogen adsorption — application of the Horvath-Kawazoe approach.
Journal of Materials Science,
Vol. 29,
Issue. 18,
p.
4927.
Chevalier, S.
Franck, R.
Lambert, J.F.
Barthomeuf, D.
and
Suquet, H.
1994.
Characterization of the porous structure and cracking activity of Al-pillared saponites.
Applied Catalysis A: General,
Vol. 110,
Issue. 2,
p.
153.
Diano, W.
Rubino, R.
and
Sergio, M.
1994.
Al-pillared montmorillonite: Preparation from concentrated slurries of homoionic Ca clay, characterization and thermal stability.
Microporous Materials,
Vol. 2,
Issue. 3,
p.
179.
Bergaoui, Latifa
Lambert, Jean-François
Franck, Raymonde
Suquet, Hélène
and
Robert, Jean-Louis
1995.
Al-pillared saponites. Part 3.—Effect of parent clay layer charge on the intercalation–pillaring mechanism and structural properties.
J. Chem. Soc., Faraday Trans.,
Vol. 91,
Issue. 14,
p.
2229.
Sánchez, Alberto
and
Montes, Mario
1998.
Influence of the preparation parameters (particle size and aluminium concentration) on the textural properties of Al-pillared clays for a scale-up process.
Microporous and Mesoporous Materials,
Vol. 21,
Issue. 1-3,
p.
117.
Molis, E.
Thomas, F.
Faisandier, K.
and
Bihannic, I.
2001.
Structural collapse of Al13- intercalated montmorillonite by Na- salicylate solutions.
Clay Minerals,
Vol. 36,
Issue. 3,
p.
335.
Sergio, M.
Cardozo, S.
Froche, C.
Bentancor, M.
Musso, M.
Medina, J.
and
Diano, W.
2003.
2001. A Clay Odyssey.
p.
639.
Li, Jianfa
Li, Yimin
and
Lu, Jinhong
2009.
Adsorption of herbicides 2,4-D and acetochlor on inorganic–organic bentonites.
Applied Clay Science,
Vol. 46,
Issue. 3,
p.
314.
Kushwaha, S.
Soni, H.
Ageetha, V.
and
Padmaja, P.
2013.
An Insight Into the Production, Characterization, and Mechanisms of Action of Low-Cost Adsorbents for Removal of Organics From Aqueous Solution.
Critical Reviews in Environmental Science and Technology,
Vol. 43,
Issue. 5,
p.
443.
Nateghi, Firoozeh
Shon, Ho Kyong
and
Khabbaz, Hadi
2014.
Development of a new poly silicate ferric coagulant and its application to coagulation-membrane filtration hybrid system in wastewater treatment.
Desalination and Water Treatment,
Vol. 52,
Issue. 4-6,
p.
663.
Laird, D. A.
and
Dowdy, R. H.
2015.
Quantitative Methods in Soil Mineralogy.
p.
236.
Martinez, J.M.
Volzone, C.
and
Garrido, L.B.
2017.
Evaluation of polymeric Al-modified bentonite for its potential application as ceramic coating.
Applied Clay Science,
Vol. 149,
Issue. ,
p.
20.
Şans, Bala Ekinci
Güven, Onur
Esenli, Fahri
and
Çelik, Mehmet S.
2017.
Contribution of cations and layer charges in the smectite structure on zeta potential of Ca-bentonites.
Applied Clay Science,
Vol. 143,
Issue. ,
p.
415.
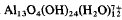 and higher molecular weight hydroxy-Al species with homoionic Na- and Ca-montmorillonite have been studied by measuring adsorption of the hydroxy-Al on to the clay, turbidity of the resulting suspensions, electrokinetic potential, and d(001) basal spacing. The isolated “Al13” ions are adsorbed according to a cation-exchange process which causes flocculation of the tactoids at low concentrations. At higher concentrations, the adsorption of either isolated “Al13” or/and higher molecular weight species is mainly responsible for the dispersion of clay particles with a net positive surface charge (ζ ∼ + 50 mV). Consequently, the tactoids are destroyed.
and higher molecular weight hydroxy-Al species with homoionic Na- and Ca-montmorillonite have been studied by measuring adsorption of the hydroxy-Al on to the clay, turbidity of the resulting suspensions, electrokinetic potential, and d(001) basal spacing. The isolated “Al13” ions are adsorbed according to a cation-exchange process which causes flocculation of the tactoids at low concentrations. At higher concentrations, the adsorption of either isolated “Al13” or/and higher molecular weight species is mainly responsible for the dispersion of clay particles with a net positive surface charge (ζ ∼ + 50 mV). Consequently, the tactoids are destroyed.

