Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Schwertmann, Udo
and
Wolska, Emilia
1990.
The Influence of Aluminum on Iron Oxides. XV. Al-for-Fe Substitution in Synthetic Lepidocrocite.
Clays and Clay Minerals,
Vol. 38,
Issue. 2,
p.
209.
Webb, J.
Evans, L. A.
Kim, K.-S.
St. Pierre, T. G.
and
Macey, D. J.
1991.
Mechanisms and Phylogeny of Mineralization in Biological Systems.
p.
283.
Zhang, Y.
Charlet, L.
and
Schindler, P.W.
1992.
Adsorption of protons, Fe(II) and Al(III) on lepidocrocite (γ-FeOOH).
Colloids and Surfaces,
Vol. 63,
Issue. 3-4,
p.
259.
Banin, A.
Ben‐Shlomo, T.
Margulies, L.
Blake, D. F.
Mancinelli, R. L.
and
Gehring, A. U.
1993.
The nanophase iron mineral(s) in Mars soil.
Journal of Geophysical Research: Planets,
Vol. 98,
Issue. E11,
p.
20831.
Herbert, Roger B.
1995.
Precipitation of Fe oxyhydroxides and jarosite from acidic groundwater.
GFF,
Vol. 117,
Issue. 2,
p.
81.
Gasser, U. G.
Jeanroy, E.
Mustin, C.
Barres, O.
Nüesch, R.
Berthelin, J.
and
Herbillon, A. J.
1996.
Properties of synthetic goethites with Co for Fe substitution.
Clay Minerals,
Vol. 31,
Issue. 4,
p.
465.
Herbert, Roger B.
1997.
Properties of Goethite and Jarosite Precipitated from Acidic Groundwater, Dalarna, Sweden.
Clays and Clay Minerals,
Vol. 45,
Issue. 2,
p.
261.
Tuhela, Laura
Carlson, Liisa
and
Tuovinen, Olli H.
1997.
Biogeochemical transformations of Fe and Mn in oxic groundwater and well water environments.
Journal of Environmental Science and Health . Part A: Environmental Science and Engineering and Toxicology,
Vol. 32,
Issue. 2,
p.
407.
Lin, Zhixun
1997.
Mobilization and retention of heavy metals in mill-tailings from Garpenberg sulfide mines, Sweden.
Science of The Total Environment,
Vol. 198,
Issue. 1,
p.
13.
Barham, R. Joseph
1997.
Schwertmannite: A unique mineral, contains a replaceable ligand, transforms to jarosites, hematites, and/or basic iron sulfate.
Journal of Materials Research,
Vol. 12,
Issue. 10,
p.
2751.
Krishnamurti, G. S. R.
Violante, A.
and
Huang, P. M.
1998.
Influence of montmorillonite on Fe(II) oxidation products.
Clay Minerals,
Vol. 33,
Issue. 2,
p.
205.
Scheinost, A. C.
Chavernas, A.
Barrón, V.
and
Torrent, J.
1998.
Use and Limitations of Second-Derivative Diffuse Reflectance Spectroscopy in the Visible to Near-Infrared Range to Identify and Quantify Fe Oxide Minerals in Soils.
Clays and Clay Minerals,
Vol. 46,
Issue. 5,
p.
528.
Wang, M. K.
Chang, C. M.
Cheng, Y. W.
Houng, K. H.
and
Chiang, H. C.
1999.
COMPARISON OF SYNTHETIC AND SOIL Al-SUBSTITUTED LEPIDOCROCITE.
Soil Science,
Vol. 164,
Issue. 5,
p.
311.
Singh, Balwant
Wilson, M. J.
McHardy, W. J.
Fraser, A. R.
and
Merrington, G.
1999.
Mineralogy and chemistry of ochre sediments from an acid mine drainage near a disused mine in Cornwall, UK.
Clay Minerals,
Vol. 34,
Issue. 2,
p.
301.
Scheinost, A.C.
and
Schwertmann, U.
1999.
Color Identification of Iron Oxides and Hydroxysulfates.
Soil Science Society of America Journal,
Vol. 63,
Issue. 5,
p.
1463.
Cumplido, Jesús
Barrón, Vidal
and
Torrent, José
2000.
Effect of Phosphate on the Formation of Nanophase Lepidocrocite from Fe(II) Sulfate.
Clays and Clay Minerals,
Vol. 48,
Issue. 5,
p.
503.
Chabbi, Abad
2000.
Naturschutz in Bergbaufolgelandschaften.
p.
331.
Yapp, Crayton
2001.
Rusty Relics of Earth History: Iron(III) Oxides, Isotopes, and Surficial Environments.
Annual Review of Earth and Planetary Sciences,
Vol. 29,
Issue. 1,
p.
165.
Dold, Bernhard
and
Fontboté, Lluis
2001.
Element cycling and secondary mineralogy in porphyry copper tailings as a function of climate, primary mineralogy, and mineral processing.
Journal of Geochemical Exploration,
Vol. 74,
Issue. 1-3,
p.
3.
Roldán, R.
Barrón, V.
and
Torrent, J .
2002.
Experimental alteration of vivianite to lepidocrocite in a calcareous medium.
Clay Minerals,
Vol. 37,
Issue. 4,
p.
709.
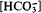 in solution and with decreasing average oxidation rate (AOR). These two parameters explained 81% of the variation of Lp/(Lp + Gt)). At a given
in solution and with decreasing average oxidation rate (AOR). These two parameters explained 81% of the variation of Lp/(Lp + Gt)). At a given 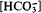 , more goethite was formed at pH 6 than at pH 7. The Lp + Gt mixtures contained 0–8 mg g−1 carbon (Ct) which could not be removed by washing. Ct reached apparent saturation at a
, more goethite was formed at pH 6 than at pH 7. The Lp + Gt mixtures contained 0–8 mg g−1 carbon (Ct) which could not be removed by washing. Ct reached apparent saturation at a 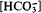 equilibrium concentration of ∼6–8 and 60–80 mmol l−1 at pH 6 and pH 7, respectively. In a plot of Ct vs. Lp/(Lp + Gt) all data fell on the same line irrespective of oxidation parameters (pH, AOR). IR spectra showed two broad bands at ∼1300 and 1500 cm−1 which can be assigned to distorted carbonate adsorbed at the goethite surface. Identical bands were also found in a young, poorly crystalline goethite formed from coal mine drainage in Ohio. It is suggested that carbonate anions direct the polymerization of the double bands of FeO3(OH)3 octahedra common to both minerals toward a corner sharing arrangement, and thereby to goethite, whereas chloride permits edge-sharing as in lepidocrocite.
equilibrium concentration of ∼6–8 and 60–80 mmol l−1 at pH 6 and pH 7, respectively. In a plot of Ct vs. Lp/(Lp + Gt) all data fell on the same line irrespective of oxidation parameters (pH, AOR). IR spectra showed two broad bands at ∼1300 and 1500 cm−1 which can be assigned to distorted carbonate adsorbed at the goethite surface. Identical bands were also found in a young, poorly crystalline goethite formed from coal mine drainage in Ohio. It is suggested that carbonate anions direct the polymerization of the double bands of FeO3(OH)3 octahedra common to both minerals toward a corner sharing arrangement, and thereby to goethite, whereas chloride permits edge-sharing as in lepidocrocite.

