Bentonites can be used in repositories for high-level radioactive waste (Neretnieks, Reference Neretnieks1978; Dohrmann et al., Reference Dohrmann, Kaufhold, Lundqvist, Bergaya and Lagaly2013a), but to do this they need to maintain their favourable properties such as their swelling and sorption capacity against heat impacts in an engineered barrier system (EBS), predominantly to ensure that diffusion is the dominant transport mechanism (Sellin & Leupin, Reference Sellin and Leupin2013). In some countries, the maximum thermal load that bentonite may be exposed to is already predetermined in disposal concepts. In other countries, this parameter is still under discussion. Accepting higher temperatures of the canister at the metal/bentonite interface would enable the disposal of higher volumes in the canister while the space demands of the entire repository would be reduced.
Examples of high-temperature concepts in clay host rock include ventilation systems that allow a maximum backfill inner-surface temperature of close to 300°C (Greenberg et al., Reference Greenberg, Wen and Buscheck2013), with the backfill composed of bentonite and sand. Increased temperatures will impact both the bentonites used in the EBS as well as the host rock. In such a setting, bentonites in EBSs would be closer to the canisters and accordingly subjected to higher temperatures than the host rock. EBS materials would, in this scenario, see reductions in some of their favourable properties such as sorption and swelling, and they act as a kind of sacrificial material in the hottest parts. In the cooler parts, EBS materials would maintain their original characteristics. Thus, EBS design has to take the expected temperatures into account while also considering the nature of the host rock.
In the Finnish and Swedish KBS-3 concept for crystalline rock, the maximum temperature allowed was set to <100°C to avoid loss of swelling capacity of smectites (Sellin & Leupin, Reference Sellin and Leupin2013). The concept was tested in a full-scale prototype repository in situ in Äspö, Sweden (Johannesson et al., Reference Johannesson, Börgesson, Goudarzi, Sandén, Gunnarsson and Svemar2007). Under these comparatively low temperatures, Dohrmann & Kaufhold (Reference Dohrmann and Kaufhold2014) barely detected any clay mineralogical changes at distances >1 cm from contact with the heater. Only the cation-exchange capacity (CEC) decreased slightly more in bentonite blocks that were subjected to higher temperatures compared to other blocks; however, the changes were only marginally outside of analytical error. In clay-based repositories, where the host rock is considered to be the main barrier, higher temperatures in contact with bentonite buffer materials can be applied (Sellin & Leupin, Reference Sellin and Leupin2013). However, temperatures >100°C may lead to boiling in cases of pressure relief in the system (e.g. Greenberg et al., Reference Greenberg, Wen and Buscheck2013). This could occur through cracks in the host rock and is more probable in the case of crystalline rocks compared to clay host rocks. Boiling was observed in the upper part of the second alternative buffer material test (ABM-2; Table 1; Dohrmann & Kaufhold, Reference Dohrmann and Kaufhold2017) while investigating the integrity of compacted bentonites as well as pellets and marine clays assembled in cages. These latter parts of the barrier were possibly not tight enough to prevent pressure loss. The pressure loss led to boiling, which in turn caused significant precipitation of halite and disintegration of some of the blocks. As a result, 100°C can be considered a reasonable temperature limit during the early phase of installation because the pressure is not high enough to raise the boiling point of water. With additional sealing or encapsulation in a tight host rock, the temperature, however, could also be higher.
Table 1. Overview of real- and medium-scale tests investigated and discussed in the present study.

HRL = hard rock laboratory; URL = underground rock laboratory.
In sum, research to date suggests that it appears reasonable to fix the maximum canister temperature to <100°C in cases of crystalline host rocks. In cases of other host rock materials, in which pressure relief caused by cracks is less likely to occur, other geochemical reactions that take place at a specific canister temperature could provide the basis for the maximum allowable temperature. As an example, the dehydroxylation temperature of smectite, which starts at ~300°C (Kuligiewicz & Derkowski, Reference Kuligiewicz and Derkowski2017), would be the ultimate temperature at which smectites degrade. Goethite, which can be present as a bentonite admixture, would dehydroxylate at 300°C. At lower temperatures, dehydration of smectite interlayers is observed, starting at <100°C ranging up to 200°C. These reactions, however, were only observed in open systems, in which water was able to leave the clay surface. In an intact EBS, water transport is limited, and one would rather observe equilibration at a temperature-dependent humidity level (e.g. (Fernandez et al., Reference Fernández, Kaufhold, Sánchez-Ledesmaa, Reya, Melóna and Robredoa2018). This will strongly depend on the initial water content of the bentonite, as shown experimentally for bentonite–halite mixtures at elevated temperatures (Kaufhold et al., Reference Kaufhold, Stührenberg and Dohrmann2009). Moreover, dehydration does not cause the structural breakdown of smectite (as dehydroxylation does), but it is known to cause some loss of swelling capacity, presumably due to cation fixation in the interlayer of smectite (Endell, Reference Endell1939; Kaufhold & Dohrmann, Reference Kaufhold and Dohrmann2010a). The degree to which this effect might be reversible is not yet known. The most important reactions with respect to the EBS in water-saturated systems might be dissolution/precipitation processes. These reactions often follow the Arrhenius law, and in the case of most minerals present in bentonites, solubility increases with increasing temperature, thus accelerating the alteration process. Lowering the temperature will decrease the reaction rate, but the principal reactions would still occur. Therefore, dissolution/precipitation processes alone are not suitable to set a maximum canister temperature. To derive a safe maximum canister temperature, all relevant physicochemical processes that could impact the integrity of the barrier at a given temperature have to be considered simultaneously. Currently, this can only be achieved through numerical modelling, which has been shown to provide a useful framework to integrate, quantify and, ultimately, better understand the thermally induced, coupled chemical and mineralogical reactions and their impacts on solute migration within barrier systems. Modelling of EBS designs, however, comes with unique challenges, as it relies on accurate thermodynamic datasets that have to cover a larger range of clay mineralogy.
Thermally induced chemical and mineralogical reactions in bentonites have been investigated both in the laboratory and after sampling of long-term deposition tests (large or medium scaled; Table 1). In the laboratory, it is possible to control the amount of water and the composition of the water (if present), neither of which is possible in deposition tests. It is, for instance, possible to investigate the integrity of bentonites under dry conditions at the laboratory scale. This led to partial cation fixation and decreases in CEC in experiments reported, for example, by Endell (Reference Endell1939), Hofmann & Klemen (Reference Hofmann and Klemen1950) and Kaufhold & Dohrmann (Reference Kaufhold and Dohrmann2010a). In these laboratory tests, water was largely absent. In an actual repository, it will be difficult to reach such dry conditions both because of the prevailing humidity and because industrially produced bentonites will be used, which will contain at least a few mass% of water.
Similarly, illitization can be investigated in the laboratory, which is barely observed in larger-scale deposition tests. Processes that are restricted to interfaces can also be investigated appropriately at the laboratory scale, as the relevant components can be mixed as powders (e.g. cement and bentonite or iron and bentonite), which then enables bulk sample analysis. However, large- or medium-scale deposition tests allow thermal gradients to evolve, which trigger hydrogeochemical reactions with gradients not observable in laboratory experiments (e.g. Mota-Heredia et al., Reference Mota-Heredia, Cuevas, Ruiz, Ortega, Torres, Turrero and Fernández2023). In addition, the impacts of low water/rock ratios, which are typical of an actual repository, are rarely reported in laboratory studies and generally require the use of natural analogues. Both types of experiments have their specific value and should both be considered when discussing thermally induced reactions in bentonites.
In the present study, data and results are discussed that were derived from the authors’ analysis of large-scale tests and their respective materials. An overview of the tests that were analysed and reported before and are discussed again in the present study is provided in Table 1. Thereby, the dimensions of real-scale tests depend on the concept employed, but the diameter of the heater is typically in the range of 1 m. The ABM medium-scale tests consisted of a 10 cm-diameter heater surrounded by 30 cm compacted bentonite rings.
The most significant hydrothermally induced reactions of bentonites identified through laboratory and heated large-scale deposition tests are:
• Cation exchange
• Decrease or increase of CEC (affecting swelling and hydraulic properties)
• Dissolution/precipitation (redistribution) of (partly) soluble phases as carbonates and sulfates and sometimes even of silicates such as cristobalite or zeolite
• Oxidation of pyrite
• Mg enrichment at the heater
• Formation of corrosion products at the heater contact (Fe-silicate or Fe-carbonate, depending on carbonate and Si abundance)
• Dissolution of smectite and precipitation of secondary silicates both at the iron (carbon steel)/bentonite and cement/bentonite interface
The present paper aims to summarize thermally induced and thermally affected reactions in bentonites, as identified in laboratory experiments, medium- to large-scale deposition tests and natural analogue studies, and to discuss current challenges in integrating these into numerical modelling frameworks. Physicochemical properties such as swelling and hydraulic conductivity (amongst others) are also affected by temperature (e.g. Kale & Ravi, Reference Kale and Ravi2018), but these are not the subject of the present review.
Cation exchange
All large-scale deposition tests in crystalline rock that reached full water saturation showed significant cation exchange caused by uptake of ambient groundwater from the host rock. Following glaciation cycles, groundwater chemistry in crystalline rocks at repository depth will change over time, with the lowest electrolyte concentration occurring after melting of the ice shield. A bentonite buffer in a repository will interact with the groundwater in such a way that the cation exchange population will constantly re-equilibrate with the cations in the groundwater. Interaction of groundwater with a relatively high salinity – as, for example, at Äspö, Sweden (Na–Ca–Cl type; e.g. Muurinen, Reference Muurinen2006) – with bentonite may differ from interaction at other test sites exhibiting lower salinities, which can result from post-glacial phases. A lower salinity of the host rock water can be found in the Grimsel test site (GTS) in Switzerland (Na–Ca–HCO3–F type; Degueldre, Reference Degueldre1994). In both cases, cation exchange plays a significant role regarding mineralogical and porewater characteristics in bentonite barriers. Cation exchange occurred within the Svensk Kärnbränslehantering AB (SKB) tests with maximum canister surface temperatures of ~140°C (e.g. Karnland et al., Reference Karnland, Olsson, Dueck, Birgersson, Nilsson and Hernan-Håkansson2009; Dohrmann et al., Reference Dohrmann, Olsson, Kaufhold and Sellin2013b; Svensson & Hansen, Reference Svensson and Hansen2013; Kumpaleinen et al., Reference Kumpulainen, Kiviranta and Korkeakoski2016), and even at <100°C (Dohrmann & Kaufhold, Reference Dohrmann and Kaufhold2014), as well as in full-scale experiments at the GTS with a relatively low-salinity groundwater at <100°C (Fernandez et al., Reference Fernández, Kaufhold, Sánchez-Ledesmaa, Reya, Melóna and Robredoa2018; Kaufhold et al., Reference Kaufhold, Dohrmann, Ufer and Kober2018). Particularly interesting is the ABM test series, which used different clays with different initial cation populations. Following saturation, the cation population equilibrated with the inflowing groundwater within 1 year. As a consequence, Na-rich bentonites lost most of the exchangeable Na+ and increased in exchangeable Ca2+, whereas Ca2+-rich bentonites lost parts of the exchangeable Ca2+ and took up some exchangeable Na+ (Dohrmann et al., Reference Dohrmann, Olsson, Kaufhold and Sellin2013b; Dohrmann & Kaufhold, Reference Dohrmann and Kaufhold2017). Interestingly, blocks of the same bentonite material located at different positions within the deposition test exhibited different cation populations. This was explained partly by varying initial conditions (i.e. water saturation during heating or before heating commenced) as well as subtle differences in the temperature that each bentonite block was exposed to depending on its location in relation to the heaters. In addition, cation population differences were correlated with the adjacent host rock characteristics. Rock fractures and rock permeability to groundwater adjacent to bentonite blocks governed water inflow. Chloride in the porewaters of different blocks equilibrated between the neighbouring blocks, starting from very different initial chloride concentrations in the different reference materials.
In summary, one can state that the cation exchange in the ABM tests was a rapid process. Considering the performance of a particular bentonite, the chemical composition of the porewater appears to be more relevant than the initial cation population of the smectites.
Cation-exchange reactions are commonly included in geochemical reaction models, and their computation according to the Gaines–Thomas, Gapon, Vanselow and Rothmund–Kornfeld conventions, allowing for both equilibrium and kinetically controlled exchange reactions, is routine (e.g. Parkhurst & Appelo, Reference Parkhurst and Appelo1999, Reference Parkhurst and Appelo2013). In barrier systems, the exchange reactions are limited by solute transport rates (e.g. the diffusion rate of solutes into the barrier). Modelling of ion-exchange reactions in diverse clays and bentonites has been reported – for example, onto MX80 bentonites (e.g. Chaparro et al., Reference Chaparro, Finck, Metz and Geckeis2021), Na-, K- and Ca-smectites (Missana et al., Reference Missana, Benedicto, García-Gutiérrez and Alonso2014), biotite (Kyllönen et al., Reference Kyllönen, Hakanen, Lindberg, Harjula, Vehkamäki and Lehto2014) and pure illites (e.g. Liu et al., Reference Liu, Zachara and Smith2004; Cherif et al., Reference Cherif, Martin-Garin, Gérard and Bildstein2017), as well as natural argillaceous rocks such as Boom Clay or Opalinus Clay (e.g. Bradbury & Baeyens, Reference Bradbury and Baeyens2000) and synthetic Na-saturated smectites (Ferrage et al., Reference Ferrage, Sakharov, Michot, Delville, Bauer and Lanson2011) for a range of radionuclides and other solutes.
Single-exchanger site models (Wallis et al., Reference Wallis, Idiart, Dohrmann and Post2016; Chaparro et al., Reference Chaparro, Finck, Metz and Geckeis2021) as well as multi-site numerical representations of clay minerals have been employed (Liu et al. Reference Liu, Zachara and Smith2004; Tournassat et al., Reference Tournassat, Gailhanou, Crouzet, Braibant, Gautier and Lassin2007; Fuller et al. Reference Fuller, Shaw, Peacock, Trivedi, Small, Abrahamsen and Burke2014). Multi-site ion-exchange models thereby simulate different types of ion-exchange sites on clays, including basal planes on crystal surfaces and interlayer sites on crystal edges. Notably, the number of interlayer sites (~80–90% of the CEC depending on pH; Vogt & Köster, Reference Vogt and Köster1978; Kaufhold & Dohrmann, Reference Kaufhold and Dohrmann2013; Christidis et al., Reference Christidis, Chryssikos, Derkowski, Dohrmann, Eberl, Joussein and Kaufhold2023) exceeds the number of edge sites. All models require representative exchange constants for the respective ion-exchange reactions. While some authors have employed fixed exchange constants in accordance with readily available thermodynamic databases (Chaparro et al., Reference Chaparro, Finck, Metz and Geckeis2021), many studies determined selectivity coefficients based on retention experiments of solutes onto clay minerals under specific pH values, ionic strengths and radionuclide concentrations. This guarantees a consistent set of coefficients for the simulation under the specific laboratory or field study conditions (e.g. Kyllönen et al., Reference Kyllönen, Hakanen, Lindberg, Harjula, Vehkamäki and Lehto2014; Missana et al., Reference Missana, Benedicto, García-Gutiérrez and Alonso2014; Baborová et al., Reference Baborová, Vopálka and Červinka2018).
A survey of experimental data regarding cation-exchange isotherms for clays and related materials, which includes temperature-dependent data, is presented in Bruggenwert & Kamphorst (Reference Bruggenwert, Kamphorst and Bolt1981). Apart from material-specific data and chemical information about the liquid phase, these authors present selectivity coefficients and, if available, free energies, enthalpies and entropies of the exchange reactions. In addition to the references presented in Bruggenwert & Kamphorst (Reference Bruggenwert, Kamphorst and Bolt1981), relevant materials, including kaolinite, bentonite, soils and vermiculite, have been studied by Assad et al. (Reference Assad, Sabet and Srivastava1981), Doula et al. (Reference Doula, Ioannou and Dimirkou1995), Udo (Reference Udo1978), Inoue (Reference Inoue1984) and Itälä & Muurinen (Reference Itälä and Muurinen2012). Note that this short list of references is certainly not exhaustive, but, together with the aforementioned tabulation, it provides an overview of the available data. These studies demonstrate that selectivity coefficients depend on the exchanger composition, the ambient temperature and the solution chemistry. The intricate interplay of these factors hinders a systematic discussion of the data at hand because a variety of materials have been studied in various solution chemistries and at different temperature intervals. The interested reader can consult Bruggenwert & Kamphorst (Reference Bruggenwert, Kamphorst and Bolt1981) as well as Maes & Cremers (Reference Maes, Cremers and Bolt1981) for more information.
Considering the thermodynamic data (exchange constants, free energy, enthalpy and entropy of exchange), some qualitative features that are present in the majority of data in Bruggenwert & Kamphorst (Reference Bruggenwert, Kamphorst and Bolt1981) can be highlighted. Considering the homovalent exchange of monovalent ions, it is noted that the enthalpic and entropic contributions to the free energy of exchange both appear to be negative, with the resulting free energy then negative as well. In systems where the initially adsorbed cation is monovalent and the one to be adsorbed is divalent, the situation becomes somewhat more complicated. For initially adsorbed sodium and a divalent exchanger ion, the enthalpy and entropy become positive quantities; however, the free energy remains negative. The total free energy of exchange appears to be entropically dominated in these systems, indicating that one can expect a notable modification of the exchange equilibrium with sufficiently large temperature intervals. If the initially adsorbed cation is either ammonium, potassium or caesium and the exchange ion is divalent, enthalpy and entropy remain positive, but now the free energy of exchange can become positive. These qualitative features apply to the majority of specified systems in cases of alkali, earth alkali and NH4+ ions. For systems involving trivalent ions, the authors were not able to find values for the enthalpy and entropy of exchange.
From this rather short and incomplete discussion of temperature-dependent cation exchange, it is evident that even establishing a consistent database of exchange constants is rather difficult. Systematic studies on well-defined materials under relevant conditions (solution chemistry and temperature) will be necessary to establish consistent exchange constants and associated thermodynamic parameters. In view of the complex mineralogical composition of barriers and host rocks (both of which probably have mixed initial cation populations) and the complex nature of groundwater, critical testing of such a database is mandatory.
Cation fixation
Clay scientists were aware of the fact that the CEC of bentonites can be reduced by thermal treatment before large-scale deposition tests were available. Long-term laboratory tests (Kaufhold & Dohrmann, Reference Kaufhold and Dohrmann2010a) showed that exchangeable cations can be fixed by the smectite structure, which means that these cations neither are exchangeable nor hydratable anymore (e.g. Mackenzie, Reference Mackenzie, Rosenquist and Graff-Petersen1963; Inoue, Reference Inoue1983). After heating at 90°C for 1.5 years, ~9% of the cations of 38 bentonites were fixed, which means that they were not exchangeable any more using the standard CEC routine (2 h shaking with Cu-trien). After 4.5 years at 120°C, 14% of the cations were fixed (non-exchangeable). Hence, only 5% more cations were fixed despite increasing the temperature by 30°C and despite a threefold increase in time (Kaufhold & Dohrmann, Reference Kaufhold and Dohrmann2010a). These numbers indicate that the process of cation fixation may be limited. In addition, the prerequisite for cation fixation is a dry environment, which is unlikely to be representative of an actual repository because of the humidity in the repository and the fact that industrially produced bentonites contain at least a few mass% of water. However, in some concepts, the bentonite will be installed with lower water contents (Sellin & Leupin, Reference Sellin and Leupin2013). The advantage of using dry bentonite is that higher dry densities can be produced with the same compaction load. In such systems, cation fixation may be important at the beginning when it is comparably dry. Under wet conditions, which will prevail later on, cation fixation will be less important.
Cation fixation can be determined by cation-exchange experiments in which ideally both the CEC and the exchangeable cations are measured. According to Gu et al. (Reference Gu, Wang, Minc and Ewing2001), who used transmission electron microscopy (TEM) to detect amorphization in the high-vacuum environment required for TEM, a decrease of the CEC was observed only at >400°C, which indicates that precise CEC measurements are required in order to determine cation fixation much below 400°C.
The mechanism(s) leading to this cation fixation are not well understood to date, and they probably differ depending on the type of cation and possibly the crystal chemistry or the layer charge density of smectites. Because of its low hydration energy, K+ is believed to enter the ditrigonal cavities where it can be fixed (Horvath & Novak, Reference Horváth, Novák and Bailey1976). Small cations such as Li+ and Mg2+ could theoretically migrate into the dioctahedral vacancies of the octahedral sheet, but this is still under discussion (Kaufhold et al., Reference Kaufhold, Dohrmann and Ufer2016). Ca2+ and Na+ are also fixed by thermal treatment (e.g. Kaufhold & Dohrmann, Reference Kaufhold and Dohrmann2010a), although they are apparently too large to migrate into the octahedral vacancies. They hence are supposed to remain within the ditrigonal cavities of the tetrahedral sheet, similarly to fixed K+. Eberl & Hower (Reference Eberl and Hower1977) observed Na+ fixation along with formation of paragonite, indicating dissolution and precipitation. Hofman & Klemen (Reference Hofmann and Klemen1950) also observed decreasing amounts of exchangeable Ca2+ and Na+ upon heating, but the temperatures were much larger than those relevant for a repository of most concepts (>300°C). According to Weiss & Koch (Reference Weiss and Koch1961), cation fixation depends on cation size and octahedral vacancies. Notably, cation fixation was observed if the samples were heated under dry conditions. In addition, Mg2+ could only be fixed under dry conditions (Kaufhold et al., Reference Kaufhold, Dohrmann and Ufer2016). One of the remaining open questions is whether fixed cations can be rehydrated using higher temperatures and excess water, which remains to be investigated in future experiments.
At the contact with the heater of large-scale deposition tests, both decreases and increases of the CEC have been observed (Dohrmann & Kaufhold, Reference Dohrmann and Kaufhold2017), which is particularly interesting regarding the ABM tests where different bentonites, which were exposed to similar conditions, exhibited different reactions (Fig. 1). The CEC decrease could be explained by cation fixation. The CEC increase, however, is more difficult to explain.

Figure 1. Bar graphs indicating percentage CEC differences of the reacted samples at the end of the test for the 2–8 cm samples (averages) for all bentonite blocks in comparison to the reference materials (REF) of the ABM-2 test (Dohrmann & Kaufhold, Reference Dohrmann and Kaufhold2017). Note that negative values indicate a CEC decrease. From Dohrmann & Kaufhold (Reference Dohrmann and Kaufhold2017). Reproduced with kind permission of The Clay Minerals Society, publisher of Clays and Clay Minerals.
A CEC decrease can be explained either by cation fixation, by smectite alteration (dissolution and precipitation as a non-swelling phase), by pH change caused by cation exchange or through pyrite oxidation and/or by dilution caused by the precipitation of other phases (addition of Fe minerals as an example would lead to a decrease in the smectite content). An increase in the CEC would indicate an increase in the smectite content, which is not likely to occur but cannot be excluded. The increase, however, could be explained by ‘reactivation’ or ‘liberation’ of previously fixed cations caused by water and elevated temperature or by an increased pH value caused by exchange of Ca2+ for Na+ (interlayer Na+ leads to higher pH values in equilibrium with a solution; Kaufhold et al., Reference Kaufhold, Dohrmann, Koch and Houben2008).
One could assume that the same bentonites would show the same trend in the ABM tests (either decrease or increase in the CEC). However, this was only partially what was observed. The MX80 blocks both at the top and the bottom of the experiment all showed a marked decrease in CEC, accounting for ~10% of the initial value. However, in block 14, which was installed in the central part and manufactured from MX80 granulates, an increase in the CEC was observed (Fig. 1). Other bentonites also showed marked differences between blocks of the same material installed at different locations within the ABM tests. This indicates that locally varying conditions were more important with respect to the decrease or increase in CEC than the type of bentonite.
The presence of water in combination with heat, leading to partial smectite dissolution, could possibly explain why the CEC of the bentonite at the contact with the heater in large-scale tests sometimes increases and sometimes decreases. Accordingly, the most important parameter determining either CEC increase or CEC decrease would be water content, followed by the amount of initially fixed cations (the CEC could not increase because of rehydration of fixed cations if the smectite did not contain any fixed cations in the reference material). The amount of fixed cations is suspected to be related to the layer charge density in such a way that high-charge smectites contain more fixed cations than low-charge ones (Kaufhold et al., Reference Kaufhold, Dohrmann, Stucki and Anastácio2011).
In summary, a portion of all common interlayer cations can be fixed under dry conditions at elevated temperatures, rendering them non-exchangeable. The fixed cations either reach an octahedral vacancy (if they are small enough) or reside within the ditrigonal holes. As the process of cation fixation and possible cation re-liberation is still under discussion, there are insufficient data to include this phenomenon in numerical modelling codes.
Oxidation of pyrite
Claystones as well as some bentonites were formed under anaerobic conditions. Under these conditions, pyrite forms upon (beginning) diagenesis from iron mono-sulfide precursors (e.g. Hunger & Benning, Reference Hunger and Benning2007). As soon as a claystone or bentonite is excavated, pyrite becomes instable and might oxidize, often forming either jarosite or gypsum (Uzarowicz & Skiba, Reference Uzarowicz and Skiba2011). Pyrite is considered to be particularly important for the EBS because it can cause Cu corrosion, act as microbial feed, decrease the pH and buffer the Eh. The initial pyrite alteration reaction is the oxidation of Fe-disulfide to sulfate by oxygen:
At acidic pH, ferric iron may trigger pyrite oxidation, but such low pH values are hardly expected to occur in the barrier systems because the smectites of the bentonites buffer the pH (e.g. Kaufhold et al., Reference Kaufhold, Dohrmann, Koch and Houben2008). Depending on the chemical environment, Fe2+ remains in solution, oxidizes to Fe-oxohydroxides (Bingham & Nordström, Reference Bingham and Nordstrom2000) or forms Fe-sulfate. The protons could lead to hydrolysis of aluminosilicates, hence promoting their weathering, and this may lead to dissolution of carbonates (Pye & Miller, Reference Pye and Miller1990), which in turn would affect porosity (Mazurek et al., 2011). Sulfate may precipitate as gypsum or jarosite.
At a molecular level, insight into this reaction was gained from density functional theory modelling, which showed the different activation energies of the various steps of the oxidation (Dos Santos et al., Reference Dos Santos, de Mendonça Silva and Duarte2016). The mechanism was recently further discussed by Feng et al. (Reference Feng, Tian, Huang, Ding and Yin2019), who stated that the mechanism of pyrite oxidation is still not well understood.
The oxidation of pyrite is not necessarily induced by elevated temperature but by moisture and oxygen, and hence it is not exclusively a thermally induced reaction. However, with regard to assessing the stability of bentonite (clay) barriers, this reaction has to be considered and included in geochemical modelling. In the ABM-5 test, which operated at 250°C (maximum), Kaufhold et al. (Reference Kaufhold and Dohrmann2021b) demonstrated that pyrite was oxidized in some samples but was preserved in others. Notably, the pyrite already found in the reference materials survived excavation in the mine and industrial production, as it was still present in the industrially dried and milled reference material. After the ABM test, pyrite was sometimes still present and oxidized elsewhere. In the ABM-2 test (T max = 140°C), pyrite, if it was initially present in the materials (MX80, JNB, FRI and IBE), was absent (Kaufhold et al., Reference Kaufhold, Dohrmann, Götze and Svensson2017). However, the ABM-2 test was characterized by high water ingress from the ambient rock, with precipitation of anhydrite observed in many blocks, possibly caused by boiling due to a pressure drop towards the surrounding crystalline rock (Dohrmann & Kaufhold, Reference Dohrmann and Kaufhold2017).
Pyrite stability and the resulting geochemical environments have been studied extensively, particularly in the field of acid mine drainage, for almost 40 years (e.g. Psenner, Reference Psenner1983; Moses et al., Reference Moses, Nordstrom, Herman and Mills1987; Nordstrom, Reference Nordstrom2011). Modelling the stability of pyrite relies on kinetic rate expressions. Rate laws for pyrite oxidation in air have been developed and are used in, for example, mine waste dam models for acid prediction (e.g. Jerz & Rimstidt, Reference Jerz and Rimstidt2004). Aqueous pyrite oxidation rate laws, which are applicable for saturated media, have been studied extensively by Williamson & Rimstidt (Reference Williamson and Rimstidt1994), Holmes & Crundwell (Reference Holmes and Crundwell2000) and Rimstidt & Vaughan (Reference Rimstidt and Vaughan2003) and have led to the widely accepted rate of aqueous pyrite oxidation by O2 (Equation 1):
where r pyr is the pyrite oxidation rate (mol m–2 s–1), kpyr is the rate constant (mol m–2 s–1) at 25°C, $C_{\rm O_2}$![]() is the concentration of dissolved oxygen (mol L–1) and $C_{\rm H^ + }$
is the concentration of dissolved oxygen (mol L–1) and $C_{\rm H^ + }$![]() is the concentration of protons (mol L–1).
is the concentration of protons (mol L–1).
The rate expression reveals a strong dependency on the oxygen concentration, while the effect of pH on the rate is small. The above is applicable for environments in the pH range of 3–10 and has been used in numerous geochemical modelling studies covering field and laboratory investigations. For instance, Appelo et al. (Reference Appelo, Verweij and Schäfer1998) successfully simulated the oxidation of pyrite in marine sediments. Other authors have quantified the release of trace metals following pyrite oxidation (e.g. Fakhreddine et al., Reference Fakhreddine, Lee, Kitanidis, Fendorf and Rolle2016; Wallis & Pichler, Reference Wallis and Pichler2018). Eckart & Appelo (Reference Eckert and Appelo2002) extended the rate expression to include pyrite oxidation by nitrate, while Prommer & Stuyfzand (Reference Prommer and Stuyfzand2005) included a temperature dependency using the Arrhenius equation to evaluate geochemical changes in response to temperature variation in a deep-well injection experiment into a pyritic aquifer.
Geochemical modelling as well as experimental studies show, however, that due to the diversity of physical and chemical properties in environmental systems, the pyrite oxidation rate expression has to be tailored towards specific environments. It is often observed that different sediments behave quite differently with respect to the extent of pyrite oxidation after excavation of a sample. After excavating a sample of the marine Friedland Clay (north Germany), it takes only hours until up to a few millimetres-long gypsum needles can be observed macroscopically. The same observations can be made with Opalinus Clay, showing the formation of cracks along with the growth of gypsum. Other clays can be stored for years in the laboratory atmosphere without any significant pyrite oxidation. One probable reason for the different tendencies of pyrite to oxidize is the trace elemental composition. According to Gregory et al. (2017), pyrites with a higher content of trace elements oxidize faster, but Co and Ni can stabilize the lattice. This effect can hardly be accounted for in modelling in which, however, the exposed surface area should be considered. For instance, framboidal pyrite, which consists of submicron-sized crystals (Fig. 2a) and therefore has a large surface area, oxidizes at faster rates than, for example, larger-sized pyrite grains (Fig. 2b; Rimstidt & Vaughan, Reference Rimstidt and Vaughan2003). The properties of the pyrite surface may also change and lead to a slowing of the oxidation reaction with time. An example is the reduction in oxygen diffusion into pyrite crystals through the increasing size of the oxidized shell, which progressively surrounds the unoxidized mineral core as pyrite oxidation progresses (e.g. Elberling et al., Reference Elberling, Nicholson and Scharer1994). Others have reported saturation of the pyrite mineral surface by precipitates of Fe(OH)3 and iron sulfates as oxidation progresses (Jerz & Rimmstidt, Reference Jodin-Caumon, Mosser-Ruck, Randi, Pierron, Cathelineau and Michau2004; Verron et al., Reference Verron, Sterpenich, Bonnet, Bourdelle, Mosser-Ruck and Lorgeoux2019). To account for changes in surface area or reactivity due to, for example, mineral ageing as pyrite dissolves, rate expressions frequently include a dependency of the pyrite oxidation rate on the surface area of the mineral (e.g. Appelo et al., Reference Appelo, Verweij and Schäfer1998) or employ an oxygen diffusion-controlled shrinking core model that describes the decrease in oxidation rate through an accumulating oxidation shell surrounding pyrite grains that acts as a diffusion barrier for O2 transport as time progresses (e.g. Davis & Ritchie, Reference Davis and Ritchie1986; Elberling et al., Reference Elberling, Nicholson and Scharer1994).
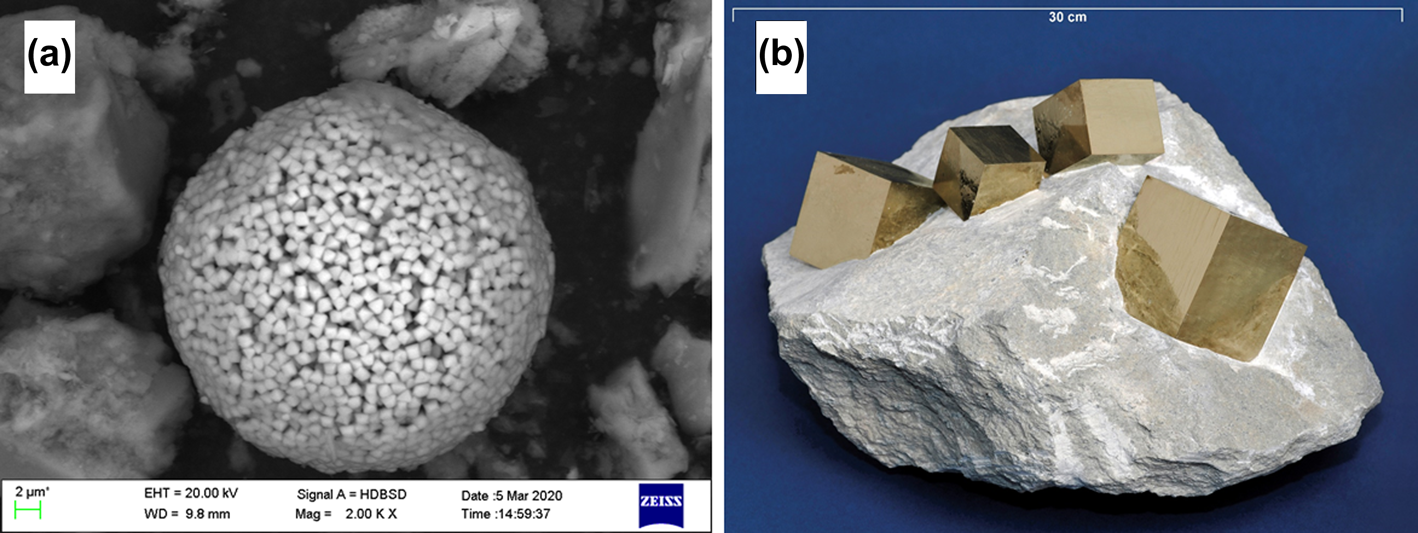
Figure 2. (a) Framboidal pyrite and (b) macroscopically perfect crystals that grew in a clay from Navajún (Spain).
In natural environments, the rate of pyrite oxidation is accelerated by microbial processes (Kappler et al., Reference Kappler, Bryce, Manson, Lueder, Byrne and Swanner2021). Therefore, some authors have introduced a microbial acceleration factor into the rate expression to simulate the impact of biological activity on pyrite oxidation rates (e.g. Kohfahl & Pekdeger, Reference Kohfahl and Pekdeger2006). Microbial processes are known to accelerate the oxidation process by several orders of magnitude under acidic pH conditions (e.g. Nordstrom & Southam, Reference Nordstrom and Southam1997). However, even at circumneutral pH, rates of microbial pyrite oxidation can be an order of magnitude higher than abiotic rates.
Correlated to microbial activity, temperature also affects the rate of pyrite oxidation (Lefebvre et al., Reference Lefebvre, Hockley, Smolensky and Gélinas2001). For moderate temperatures, the temperature dependence of pyrite oxidation is adequately described by the Arrhenius equation (Prommer & Stuyfzand, Reference Prommer and Stuyfzand2005). However, high-temperature environments may limit reaction progress due to a reduction in microbial activity as a result of cell death under higher temperatures. As a result, microbially mediated reaction rates, such as those observed for pyrite oxidation, decline at temperatures that exceed the optimum temperature for microbial activity (Lefebvre et al., Reference Lefebvre, Hockley, Smolensky and Gélinas2001; Xie et al., Reference Xie, Xiao, He, Liu and Qiu2007). As a consequence, some authors have included an inhibition factor in the pyrite oxidation rate expression for elevated temperatures (e.g. Yi et al., Reference Yi, Su, Seigneur and Mayer2021). In this context, it should be noted, that increased temperatures can be naturally achieved in reactive pyritic rock due to the exothermic nature of pyrite oxidation processes (Lefebvre et al., Reference Lefebvre, Hockley, Smolensky and Gélinas2001; Amos et al., Reference Amos, Blowes, Bailey, Sego, Smith and Ritchie2015).
Pyrite is supposed to be the main sulfide phase in both claystones and bentonites, but theoretically mono-sulfides such as mackinawite (FeS) and greigite (Fe3S4) could also exist (Hunger & Benning, Reference Hunger and Benning2007) or form at interfaces such as between bentonite and the canister. Different mono-sulfides were found after excavating bentonite from the Full-Scale Engineered Barrier Experiment (FEBEX; Fernandez et al., Reference Fernández, Kaufhold, Sánchez-Ledesmaa, Reya, Melóna and Robredoa2018).
In summary, pyrite stability depends on the exposed surface area of pyrite, which in turn depends on particle size and crystallinity and trace elemental composition, but also on temperature, oxygen concentration, internal porewater chemistry (e.g. pH, availability of ferric iron), microbiology and water content. For reasonable inclusion of pyrite oxidation into geochemical models, empirical studies on the pyrite characteristics within the employed bentonite should be conducted and existing oxidation rate expressions adjusted accordingly.
Dissolution and precipitation
Carbonates, gypsum and anhydrite
The long-term test conducted by SKB in Äspö (LOT; Table 1) was the first in situ experiment in which a redistribution of sulfate in the compacted bentonite was observed (Muurinen, in Karnland et al., Reference Karnland, Olsson, Dueck, Birgersson, Nilsson and Hernan-Håkansson2009). At the start of the experiment, gypsum was evenly distributed, with a sulfate content of 0.27 mass% throughout the blocks (dashed blue line in Fig. 3). After the test, the retrieved blocks were analysed at different distances from the heater. Samples collected near the crystalline rock showed a slightly lower sulfate content, but a maximum concentration was observed approximately in the centre of the blocks at a distance of ~4 cm. The contact sample at the Cu heater showed slightly increased sulfate values compared with the 1 cm sample, which could be explained by the formation of the corrosion product Cu(Fe)S. This redistribution of sulfate was modelled successfully based on the observed thermal and hydraulic gradients by Arcos et al. (Reference Arcos, Bruno, Benbow and Takase2000). The authors simulated the behaviour of the bentonite accessory minerals calcite, anhydrite, siderite, pyrite and quartz during the thermal stage and highlighted the relevance of the cation-exchange population of the bentonite. The authors also demonstrated that cation-exchange reactions controlled the concentration of calcium, which, in turn, controlled the precipitation and dissolution of calcite. With calcium being the dominant ion within the bentonite porewaters, anhydrite ‘migrated’ towards the clay/crystalline rock interface via dissolution and precipitation. They concluded that cation-exchange processes probably dominate the ionic inventory within the bentonite, even after 100,000 years.

Figure 3. Sulfur (determined by LECO C-S analyser; Karnland et al., Reference Karnland, Olsson, Dueck, Birgersson, Nilsson and Hernan-Håkansson2009) redistribution in the LOT A2 experiment (Table 1). The dashed blue line represents the initial sulfur content resulting from both pyrite and gypsum.
In the LOT experiment (Fig. 3), only MX80 bentonite was used. In the ABM tests, in which different bentonites with different S contents and different hydraulic properties were used, different S redistribution patterns were identified. In the first alternative buffer material test (ABM-1), S enrichment at the contact with the heater was observed for some bentonite blocks, which resulted from the precipitation of anhydrite (e.g. sample FEB, ABM-1; Kaufhold et al., Reference Kaufhold and Dohrmann2013). Other blocks, however, showed slightly decreasing S concentrations towards the host rock, and others showed unchanged concentrations. In the ABM-2 test, unchanged gypsum concentrations were found in the top and the bottom bentonite blocks, and significant anhydrite precipitation was observed where boiling occurred as a result of a local pressure relief (Dohrmann & Kaufhold, Reference Dohrmann and Kaufhold2017). It is assumed that the water vapour left the system via a leakage in the barrier and presumably the crystalline rock as well. Gypsum, in conclusion, is a rather soluble component of some bentonites and readily dissolves and precipitates within the barrier. The thermodynamic constants of gypsum are well known (e.g. Nordstrom, Reference Nordstrom2013; Shen et al., Reference Shen, Sippola, Li, Lindberg and Taskinen2019). Accordingly, the fate of gypsum in different barrier systems can be simulated on the basis of the time-dependent thermohydraulic conditions within the barrier. The results of the ABM tests are particularly valuable for the verification of gypsum dissolution and precipitation reactions and their modelling because rather different profiles were obtained for different bentonite blocks, which can be used to verify model results. According to Karnland (Reference Karnland1995), gypsum redistribution is favoured by a high buffer density, a low content of accessory minerals, a low electrolyte content in the surrounding water and a high water pressure.
Carbonates are less soluble than gypsum, but results obtained from different large-scale tests showed that carbonates can also dissolve and precipitate throughout the barrier system, similarly to gypsum. In the ABM-1 test, inorganic carbon enrichment was detected only at the contact with the Fe heater. This could, therefore, be related to the corrosion process in which sometimes siderite forms. The C needed for siderite formation may be derived from initially present carbonates in the bentonite, from C contained in the carbon steel or from CO2/HCO3– present in the (inflowing) porewater. In the ABM-2 test, in which boiling occurred in the upper part of the core, a decrease in the carbonate content was detected. In the lower part, however, calcite showed only minor variation. A special reaction was observed in ABM-5 (Kaufhold et al., Reference Kaufhold, Dohrmann, Ufer, Svensson and Sellin2021). Because of the absence of the paraffin-rich lubricant Molykote® (DuPont), which in the other tests was used to support canister emplacement, the CO2 signal derived from simultaneous thermal analysis could be unambiguously related to siderite, which formed as the calcite concentration decreased (Fig. 4). This process probably occurred in the other ABM tests as well but could not be detected unambiguously because of the presence of Molykote BR-2 Plus. The formation of siderite was restricted to the iron/bentonite interface. Several authors identified magnetite and Fe-silicate as the main corrosion products (Lantenois et al., Reference Lantenois, Lanson, Muller, Bauer, Jullien and Plançon2005; Wilson et al., Reference Wilson, Cressey, Cressey, Cuadros, Ragnarsdottir, Savage and Shibata2006a, Reference Wilson, Savage, Cuadros, Shibata and Ragnarsdottir2006b; Perronet et al., Reference Perronnet, Villiéras, Jullien, Razafitianamaharavo, Raynal and Bonnin2007; Carlsson et al., Reference Carlsson, Karnland, Rance, Smart, Snellman, Vähänen and Werme2008; Osacky et al., Reference Osacky, Ŝucha, Czímerová and Madejová2010; Kaufhold et al., 2015). In some corrosion studies, carbonates were added. These studies found siderite as a corrosion product either along with Fe-silicates and magnetite or even alone (Savoye et al., 2001; Martin et al., 2008; Romaine et al., 2013; Mendili et al., 2014). Modelling the long-term behaviour of the corrosion interface showed that siderite will be an important phase (Savage et al., Reference Savage, Watson, Benbow and Wilson2010) and that the initial Fe-silicates will recrystallize. In archaeological artefacts used as natural analogues (Saheb et al., 2010, 2013), magnetite and siderite were the dominating corrosion products.

Figure 4. Mass spectrometer (MS) curves of water (blue) and CO2 (black/grey) of the reference (ref) sample (brighter colour) and contact sample (darker colour) of block 10 from the ABM-5 test (Kaufhold et al., Reference Kaufhold, Dohrmann, Ufer, Svensson and Sellin2021). Before: composition of the ref material used to produce the blocks for the experiment; after: block material retrieved after termination of the experiment. The heating rate was 10 K min–1.
The macroscopic dissolution and precipitation kinetics of the bentonite accessory minerals carbonate, gypsum and anhydrite are routinely approximated as an equilibrium reaction because the respective reaction rates are considered ‘sufficiently fast’ compared to the minimal time resolution in the model. Thus, local equilibrium is assumed to be reached instantaneously. For instance, Samper et al. (Reference Samper, Naves, Montenegro and Mon2016) employed a multicomponent reactive transport model to study the long-term interactions of corrosion products and compacted bentonite in a high-level radioactive waste repository in crystalline rock. All mineral reactions, except for canister corrosion, were assumed to be at chemical equilibrium, including calcite and gypsum dissolution and precipitation. Numerical simulations were performed over a time horizon of 1 Ma. Samper et al. (Reference Samper, Mon and Montenegro2018) simulated the hydrochemical changes within the FEBEX in situ test, calibrated on the basis of observed gravimetric water content, dry density, temperature and major ion concentrations. The model spanned a period of 18 years and assumed calcite, anhydrite and gypsum to be at chemical equilibrium over the duration of the experiment. Using this approach, the computed major ion concentrations were broadly consistent with observed porewater concentrations, measured at radial distance from the heater/bentonite interface at the end of a first (year 2002) and second operational phase (year 2015) of the FEBEX experiment. The predicted concentrations of dissolved Ca2+ and HCO3– were thereby linked to the dissolution/precipitation of calcite and sulfate minerals. The simulated dissolved SO42− concentrations underestimated the porewater concentration data, possibly due to uncertainties in the initial amount of gypsum in the bentonite.
A similar approach was followed by Arcos et al. (Reference Arcos, Bruno, Benbow and Takase2000), simulating the interaction between bentonite buffer and groundwater over 100,000 years. Calcite, siderite, anhydrite, quartz and pyrite were considered equilibrium reactions, where the solubility constant was scaled on the basis of temperature. Sena et al. (Reference Sena, Salas and Arcos2010) simulated the long-term buffer material LOT A2 test at the Äspö Hard Rock Laboratory. The LOT A2 test lasted 6 years and consisted of a heating element encapsulated by a copper tube, which in turn was surrounded by pre-compacted Wyoming MX80 bentonite. Again, the dissolution/precipitation of calcite, anhydrite and gypsum were assumed to be equilibrium reactions, leading one to expect that these mineral phases would demonstrate very fast reactivity under the thermohydraulic and chemical conditions that prevailed during the LOT A2 test. The numerical results predicted the dissolution/precipitation of anhydrite, calcite and silica in the heated bentonite in agreement with data measured at the end of the LOT A2 test. In general, gypsum, anhydrite and carbonate reaction rates do vary with temperature. The solubility behaviour of anhydrite is similar to that of calcite and increases with decreasing temperature at low pressure (e.g. Newton & Manning, Reference Newton and Manning2005). The solubility of gypsum changes only slightly up to temperatures of ~55°C (Reiss et al., Reference Reiss, Gavrieli, Rosenberg, Reznik, Luttge, Emmanuel and Ganor2021).
If the equilibrium approach is considered inadequate, generic kinetic rate laws have been employed, which generally take the reactive surface area and saturation state of the respective mineral into account and can take the form seen in Equation 2:
where R is the overall reaction rate (mol m–3 s–1), rmineral is the rate constant (mol m–3 s–1), which is temperature dependent, A is the initial surface area of the mineral (m2), V is the solution volume (m3), m 0 is the initial moles of the solid, m is the moles of solid at a given time and n is a coefficient that takes the value of 2/3 for uniformly dissolving/growing spheres and 1/2 for cylinder-shaped grains but is generally determined empirically for different minerals as it varies as a function of initial grain-size distribution and changes in grain-size distribution and reactive site density during dissolution/precipitation (Larsen & Postma, Reference Larsen and Postma2001).
The term $\left({{m \over {m_0}}} \right)^n$![]() thereby allows for the reaction rate to account for changes in reactive surface sites during dissolution, but it may also account for changes in crystal size, ageing of the solid or armouring of reactive surfaces (Appelo & Postma, Reference Appelo and Postma2005). The term f(C) allows for the reaction rate to be scaled (e.g. depending on the departure from equilibrium) as a function of pH or solution composition (Lasaga, Reference Lasaga1998).
thereby allows for the reaction rate to account for changes in reactive surface sites during dissolution, but it may also account for changes in crystal size, ageing of the solid or armouring of reactive surfaces (Appelo & Postma, Reference Appelo and Postma2005). The term f(C) allows for the reaction rate to be scaled (e.g. depending on the departure from equilibrium) as a function of pH or solution composition (Lasaga, Reference Lasaga1998).
In summary, solubilities of gypsum, anhydrite and carbonates have been investigated extensively. Nevertheless, some uncertainties remain regarding the equilibrium constants reported in the literature. Variation in the values for equilibrium constants can result in solubility and solid-phase predictions that differ in magnitude between thermodynamic models; however, research to improve the available thermodynamic data for carbonates, gypsum and anhydrite mineral phases is ongoing (e.g. Bouchelaghem, Reference Bouchelaghem2010; Ruiz-Agudo et al., Reference Ruiz-Agudo, Putnis, Hövelmann, Álvarez-Lloret, Ibánez-Velasco and Putnis2015; Dai et al., Reference Dai, Kan, Shi, Zhang, Zhang and Yan2017; Voigt et al., Reference Voigt, Marieni, Deirdre, Gislason and Oelkers2018). Another source of model uncertainty stems from the dependence of kinetic reaction rates on reactive surface areas. Only a small percentage of the total mineral surface area may actually participate as a reactive surface area, which is difficult to estimate with precision and is often approximated (e.g. White & Peterson, Reference White, Peterson, Melchior and Bassett1990). In general, however, variations in surface area of fast-reacting phases such as calcite only impact model simulations over short timescales.
Silicates
Silicates are generally considered to be relatively insoluble. At elevated temperatures, however, minor amounts of Si, Al, Mg and Fe are known to dissolve from smectite, entering the solution. Kaufhold et al. (Reference Kaufhold, Dohrmann, Degtjarev, Koeniger and Post2019) showed that increasing temperature leads to an increasing ratio of dissolved Si/Mg. At <120°C, more Mg was dissolved compared to the stoichiometric dissolution (Mg dominated incongruent dissolution) and more Si was dissolved at temperatures >120°C, suggesting that at least at such high temperatures smectites dissolve incongruently. However, the data shown in Fig. 5 suggest that at temperatures of ~120°C smectites may dissolve congruently even at circumneutral pH – or at least Mg and Si dissolve congruently. Congruent dissolution including Al and Fe, however, could not be confirmed because of the difficulties of measuring dissolved Al and Fe (explanations given by Kaufhold et al., Reference Kaufhold, Dohrmann, Degtjarev, Koeniger and Post2019). At high pH, congruent dissolution of smectites was reported by Cama et al. (Reference Cama, Ganor, Ayora and Lasaga2000). Figure 5 shows that three bentonites (B6, B11, B16) principally show the same curves despite their rather different compositions (e.g. the high Fe content of B11). The pronounced dissolution of Si from bentonite B38 can possibly be explained by a higher quartz content.

Figure 5. Si/Mg ratio of short-term smectite dissolution tests at circumneutral pH of four different bentonites (B6, B11, B16, B38) at different temperatures. The bentonites differed with respect to their composition and cation population (Kaufhold et al., Reference Kaufhold, Dohrmann, Degtjarev, Koeniger and Post2019).
The composition of the porewater with respect to structural cations dissolved from the smectites, therefore, is supposed to be variable depending on the temperature. Smectite dissolution in large-scale tests has not been observed to date. In the ABM-1 experiment, however, the dissolution of cristobalite and clinoptilolite was observed in some contact samples. Both minerals are supposed to be less stable (more soluble) compared to feldspar, quartz or most other silicates.
The dissolution and precipitation kinetics of primary minerals such as quartz and feldspar that are present in igneous and metamorphic rocks or as detrital minerals in sedimentary rocks are generally slow. Developed rate laws commonly describe the dissolution and precipitation of primary minerals as a function of available reactive surface area and saturation state. The latter allows for the reaction rate to be scaled depending on the departure from equilibrium. The maximum rate of dissolution/precipitation occurs far from equilibrium; however, reaction kinetics slow when the solubility of the silicate mineral is being approached (Lasaga, Reference Lasaga1998). Silicate reaction rates vary with temperature, which can be approximated using the Arrhenius equation (Rimstidt & Barnes, Reference Rimstidt and Barnes1980), and they also vary with porewater composition (e.g. pH, [Al], [Mg]; e.g. Dove, Reference Dove1999).
Thermodynamic data for most primary minerals exist, providing a solid base for modelling. However, it should be noted that reaction rates for silicate minerals, which have been determined in the laboratory, have generally been found to be one or more orders of magnitude faster than those observed in the field (e.g. White & Brantley, Reference White and Brantley2003; Parry et al., Reference Parry, Hodson, Kemp and Oelkers2015). Rate variations are attributed to differences in the composition of silicates (i.e. field reaction rates reflect the dissolution/precipitation kinetics of solid solutions rather than pure end-member minerals; Maher et al., Reference Maher, Johnson N., Lammers, Torchinsky, Weaver, Bird and Brown2016), with most modelling studies adopting the simplification of treating silicates and clays as discrete end-member compositions (e.g. Wilson et al., Reference Wilson, Savage, Cuadros, Shibata and Ragnarsdottir2006b; Marty et al., Reference Marty, Fritz, Clément and Michau2010).
Weathering of primary minerals results in the formation of secondary minerals, including smectite and other clay minerals. The variation in the composition of clay minerals such as smectite is large, and as a consequence, in contrast to primary minerals, the thermodynamic properties of smectites, which govern the stability of these minerals in solution, remain largely unresolved (Gailhanou et al., Reference Gailhanou, Blanc, Rogez, Mikaelian, Kawaji and Olives2019). May et al. (Reference May, Kinniburgh, Helmke and Jackson1986), for instance, discussed the experimental difficulties in the evaluation of dissolution experiments of smectites. Nevertheless, smectite dissolution rates have been published by Cama et al. (Reference Cama, Ganor, Ayora and Lasaga2000), Golubev et al. (Reference Golubev, Bauer and Pokrovsky2006) and Rozalén et al. (Reference Rozalén, Huertas, Brady, Cama, Garcia-Palma and Linares2008); however, equilibrium achievement remains a significant issue in these solubility experiments (Blanc et al., Reference Blanc, Gherardi, Vieillard, Marty, Gailhanou and Gaboreau2021). Wilson et al. (Reference Wilson, Savage, Cuadros, Shibata and Ragnarsdottir2006b) calculated the solubilities of different Fe-smectites and derived log(K) values that varied from –12 to 33 at 25°C, while Gailhanou et al. (Reference Gailhanou, Vieillard, Blanc, Lassin, Denoyel and Bloch2017) showed that hydration reactions modified the stability of smectite by >1 log(K) unit per mole of water. The large range of values probably results from ‘uncertainties associated with the thermodynamic modelling of clay mineral stability (Lippmann, Reference Lippmann1979) and the wide variety in clay mineral composition and structure’ (Wilson et al., Reference Wilson, Savage, Cuadros, Shibata and Ragnarsdottir2006b).
Modelling codes require stability constants for the initial clay composition of the barrier system, including for smectites, as well as for any potential clay mineral end member that could form after chemical and thermal disturbances over time. With few experimental data available in the literature, current practice uses estimation methods to obtain thermodynamic data in the databases of geochemical codes for clay phases whose chemical composition is known (Blanc et al., Reference Blanc, Gherardi, Vieillard, Marty, Gailhanou and Gaboreau2021). Based on a limited number of experimental measurements, results are thereby extended to different clay mineral compositions to obtain thermodynamic datasets covering a larger range of clay mineralogy. Marty et al. (Reference Marty, Claret, Lassin, Tremosa, Blanc and Madé2015) simulated smectite dissolution and concluded that the major model uncertainty derived from the fact that incongruent dissolution of smectite had to be accounted for. In addition, the authors acknowledged the problem of determining the reactive surface of smectites (and other clay minerals), which is required for any kinetic modelling of their precipitation or dissolution. In addition to a reactive surface area and incongruent dissolution, smectite dissolution depends on temperature, pH and the degree of saturation of the solution. Accordingly, Alekseyev et al. (2007) and Marty et al. (Reference Marty, Claret, Lassin, Tremosa, Blanc and Madé2015) developed a comprehensive set of (semi-)empirical relations. This approach was also followed by Ngo et al. (Reference Ngo, Delalande, Clément, Michau and Fritz2014), who simulated the dissolution of smectite as a function of pH, employing different kinetic constants for three distinct pH ranges: acidic, neutral and alkaline porewater conditions. These approaches provide means to consider relevant effects on the dissolution rate. However, the overall complexity of describing the dissolution of smectites introduces considerable uncertainty into current modelling studies.
In summary, modelling dissolution and precipitation of the siliceous admixtures of bentonite is less complicated compared to with smectite. Improving the understanding of smectite dissolution mechanisms hence is crucial for improving modelling of long-term barrier performance.
Mg enrichment at the heater
In most of the large-scale tests in which a contact sample was analysed, at least a slight Mg increase was observed at the heater (e.g. Fig. 6). Svensson et al. (Reference Svensson, Dueck, Nilsson, Olsson, Sandén and Lydmark2011) analysed four bentonite blocks from the ABM-1 experiment that were heated to 140°C. The authors reported that in three of the four blocks a Mg gradient had developed (increasing MgO content towards the heater). Interestingly, the Mg concentration of each of the reference materials (before the experiment started) was higher than in any of the samples taken after termination of the experiment, but no contact sample was investigated in this study. By comparing different bentonites, they could exclude dissolution of carbonates such as dolomite as the Mg source. Kaufhold et al. (2013) also showed that most of the nine analysed bentonite blocks exhibited Mg increases towards the heater. In ABM-2, which was heated to 140°C, 29 out of 31 bentonite blocks were sampled. The contact of most blocks showed MgO increases of >0.1 mass%, while nine blocks showed an increase of ≥1 mass% (first 10 blocks shown in Fig. 6b). Kumpulainen et al. (Reference Kumpulainen, Kiviranta and Korkeakoski2016) confirmed this for two ABM-2 bentonite blocks (the block labelled FRI was not included here as that was a marine clay from Friedland, Germany).

Figure 6. Example of (a) a MgO profile of the FEBEX experiment (section 54 without liner) based on Kaufhold et al. (Reference Kaufhold, Dohrmann, Ufer and Kober2018) and (b) MgO profiles of the first 10 blocks of ABM-2 (Kaufhold et al., Reference Kaufhold, Dohrmann, Götze and Svensson2017). Temperature gradients (pink) were taken from Martinez et al. (Reference Martinez, Abós and García-Siñeriz2016; FEBEX) and Kaufhold et al. (Reference Kaufhold, Dohrmann, Götze and Svensson2017; ABM-2). The temperature profiles in the ABM-2 test showed some variation depending on depths. Therefore, minimum and maximum values are shown. Reference values (MgO content before the experiment) are given as bold lines assigned as ‘ref’. Higher contents of the ref materials can be explained by exchange of initially present exchangeable Mg2+.
In contrast, ABM-5, the experiment with the highest temperature of 250°C, did not exhibit a clear trend in the Mg distribution. Six blocks even showed a slight MgO decrease of >0.1 mass% towards the contact, while 15 blocks showed an increase of between 0.2 and 1.1 mass% MgO. Dohrmann & Kaufhold (Reference Dohrmann and Kaufhold2014) studied two MX80 blocks after retrieval of the prototype repository in situ experiment in Äspö, Sweden. They reported that exchangeable Mg in the buffer decreased (by cation exchange), while the MgO concentration had increased at the bentonite/Cu canister interface. A uniform MgO distribution farther away from the contact can be explained by cation exchange, which may also at least partly be the source of the Mg at the heater.
It should be noted that the Mg increase towards the heater is commonly expressed as ‘+x% MgO’ (absolute increase in mass% from the chemical analysis) and that these values depend on the area of the piece of the block used for sampling: the so-called ‘contact samples’ (all other samples were drilled). Commonly, 10–30 cm2 are required to collect 2 g of contact sample (i.e. the amount needed for mineralogical analysis). Accordingly, the available surface area for sampling will dictate the depth of sampling, with larger surfaces available for sampling requiring a reduced sampling depth. The samples collected from the FEBEX experiment had a surface of ~200 cm2 and therefore a reduced sampling depth (<0.1 mm; Fig. 6a; Kaufhold et al., Reference Kaufhold, Dohrmann, Ufer and Kober2018). The experiment itself was conducted over 18 years, which could be an additional explanation as to why the highest Mg increase was observed in this experiment. The Mg increase was often observed, but for some materials no specific Mg-rich phase was observed (e.g. LOT experiment; Kaufhold et al., Reference Kaufhold, Dohrmann, Götze and Svensson2017). In some samples from the ABM tests, however, a small increase of the d 060 reflection of the smectite and a slight increase of the 680 cm–1 infrared band were observed, which both indicate the formation of trioctahedral domains (Fig. 7). This effect has already been reported by Plötze et al. (Reference Plötze, Kahr, Dohrmann and Weber2007) and Svensson (Reference Svensson2015). Trioctahedral domains could at least theoretically form as a result of an addition reaction (Mg would enter the vacancies), or they could form as a separate trioctahedral mineral by dissolution and precipitation. Laboratory experiments showed that rather dry conditions were required to fix Mg in the vacancies (Kaufhold et al., Reference Kaufhold, Dohrmann and Ufer2016). In the ABM tests, however, such dry conditions did not exist. Therefore, it is more probable that either Mg was dissolved incongruently from smectite and precipitated as a new phase near the heater or that Mg was liberated by cation exchange and migrated to the heater. In the FEBEX contact block, Kaufhold et al. (Reference Kaufhold, Dohrmann, Ufer and Kober2018) found brucite, and Fernandez et al. (Reference Fernández, Kaufhold, Sánchez-Ledesmaa, Reya, Melóna and Robredoa2018) also observed other Mg-rich silicates such as sepiolite using an electron microscope. This indicates that the Mg increase may not be triggered by a specific reaction, instead indicating that the precipitation of Mg phases is the result of Mg diffusion towards the heater caused by the thermal and hydraulic gradients. The Mg, as explained above, may be from cation exchange and/or (incongruent) dissolution of smectite. The latter would be consistent with data published by Kaufhold et al. (Reference Kaufhold, Dohrmann, Degtjarev, Koeniger and Post2019), who showed that the Mg/Si ratio of leached smectites depends on the temperature. The experimental data and modelling performed on Mg- and Si-oxides indicated that at temperatures <120°C more Mg is liberated by dissolution of smectites, whereas at higher temperature more Si (in relation to Mg) is liberated (Fig. 5). This could lead to a concentration gradient of Mg and Si corresponding to the respective temperature. In the colder parts more Mg would be available in solution, whereas in the hotter parts more Si would be available in solution. This could lead to migration of Mg towards the heater, where it may precipitate as a Mg-rich phase. This should, however, lead to a continuous Mg gradient inside the block. However, the Mg increase is often restricted to the contact. It also would not explain why sometimes pure Mg phases form (e.g. brucite in the FEBEX experiment). In this respect, it is interesting to note that in the case of a saponite being used in the ABM-5 test, a Mg decrease at the heater was observed (Kaufhold et al., Reference Kaufhold, Dohrmann, Ufer, Svensson and Sellin2021). The material, of course, had a high initial Mg content (~16 mass% MgO).

Figure 7. (a) Increase in the d 060 intensity at ~1.54 Å (trioctahedral minerals) and (b) increase in the extinction of the 680 cm–1 infrared band, suggesting the presence of trioctahedral minerals (Kaufhold et al., Reference Kaufhold, Dohrmann, Ufer and Kober2018). REF = reference.
There is a paucity of modelling studies that report on the evolution of Mg as part of bentonite buffer simulations for radioactive waste confinement. However, some studies report on alterations in Mg content in bentonites simulated on the basis of dissolution reactions. Marty et al. (Reference Marty, Fritz, Clément and Michau2010) simulated long-term alterations of MX80 in contact with ambient geological fluids and a corroding steel canister, and they observed the dissolution of montmorillonite, which in turn resulted in elevated Mg porewater concentrations. Similarly, Savage et al. (Reference Savage, Watson, Benbow and Wilson2010) modelled Mg release from dissolving Ca-montmorillonite as part of the long-term geochemical changes of a bentonite buffer in contact with a steel canister. Subsequently, dolomite, as an Mg-rich calcium carbonate, was simulated to precipitate in proximity to the iron canister/bentonite interface. It is conceivable that bentonite dissolution and subsequent diffusion of Mg and/or thermal-induced migration towards the heater (Jodin-Caumon et al., Reference Jodin-Caumon, Mosser-Ruck, Randi, Pierron, Cathelineau and Michau2012) could lead to Mg enrichment; however, to the knowledge of the authors, this process has not been described as part of a model study to date. The possibility of predicting this reaction would largely depend on the accuracy with which smectite dissolution, including incongruent Mg dissolution from smectite, could be described as a function of temperature.
Illitization
The reaction of smectites with K+ is frequently investigated because the oil industry uses the parameter ‘illite crystallinity’ or ‘Kübler index’ to obtain information about the burial history of sediments, which in turn is important with respect to the formation of oil and gas in the deeper sediments. The basic idea behind progressive illitization is that the marine sediments, as they settled on the sea floor, contain smectite, amongst other minerals. The burial history of such marine clays starts as the amount of overlying sediment increases. The increasing load leads to compaction and the formation of a claystone or shale. With increasing temperature and time, the organic matter matures, and at certain points it forms oil and gas. Such maturation sequences are observed in basins containing marine clays. The maturity of the organic material can be correlated with the amount of illite that formed in relation to the starting material. Illitization, therefore, results from increased pressure, temperature and time, but it also depends on the availability of K+ in the basins. Without the presence of K+, smectite is preserved despite significant overload for several hundreds of millions of years, and hence illitization can be considered slow compared with the lifetime of a repository (Sellin & Leupin, Reference Sellin and Leupin2013). The reaction of smectite and K+ is special because K+ can be fixed in the ditrigonal holes of the tetrahedral sheet (because of its diameter and low hydration energy; Lagaly, Reference Lagaly, Jasmund and Lagaly1993) and because it can induce the formation of illite (Kaufhold & Dohrmann, Reference Kaufhold and Dohrmann2010b). To date, two conceptual models are considered that could explain illitization: solid-state transformation (Hower et al., Reference Hower, Eslinger, Hower and Perry1976) and dissolution and precipitation (Boles & Franks, Reference Boles and Franks1979).
The simplest pathway for conversion of smectite into a 10 Å phase would be to exchange the exchangeable cations by K+ followed by drying to remove the hydration water of K+. The resulting 10 Å peak in XRD is often misinterpreted as illite. In fact, this peak corresponds to a 2:1 layer silicate with a dehydrated interlayer but not necessarily illite, which is distinguished from smectite based on its layer charge density (0.6–0.9 eq FU–1 instead of 0.2–0.6 eq FU–1 for smectites) and the extent of Si-by-Al substitution in the tetrahedral sheet.
Illitization has been investigated in several laboratory studies (Eberl, Reference Eberl1978; Güven & Huang, Reference Güven and Huang1991; Herbert et al., Reference Herbert, Kasbohm, Moog and Henning2004; Hofmann et al., Reference Hofmann, Bauer and Warr2004; Suzuki et al., Reference Suzuki, Sazarashi, Akimoto, Haginuma and Suzuki2008). Kaufhold & Dohrmann (Reference Kaufhold and Dohrmann2010b) reacted different bentonites with KCl solutions and could distinguish different phases: real illite (no H2O swelling (= zero-water-layer state) and no cation exchange from the interlayer region), K+-exchanged smectite 1 (no H2O swelling (= zero-water-layer state) but exchangeable K+ from the interlayer region) and K+-smectite 2 (hydratable and exchangeable K+ from the interlayer region). Through XRD analysis and measuring under ‘air-dry’ conditions and with ethylene glycol (EG) solvation, one could distinguish even more phases. In most studies in which significant formation of real illite was observed, chemicals were added supporting the dissolution of smectite, such as KOH (Bauer & Velde, Reference Bauer and Velde1999) or organic acids (Schlosser et al., Reference Schlosser, Grathoff, Warr, Derkowski, Kaufhold, Schleicher and Wirth2019), indicating that smectite dissolution is important with respect to illitization. Notably, the water/solid ratios were much larger compared to repository conditions, which would have accelerated illitization in these experiments.
According to the model provided by Kaufhold & Dohrmann (Reference Kaufhold and Dohrmann2010b), real illite forms if K+, Al and Si are present in solution. In a system with excess water containing K+, smectite (depending on temperature) partly dissolves (most likely only small percentage), providing some Al and Si within the solution. If K+ is present, illite precipitates, leaving some silica in solution, which is a byproduct of real illitization (‘real’ means that the actual crystal chemical process occurred, not just a collapse of the interlayer region). As a result of illite precipitation, the solution is depleted in Al and Si, and smectite dissolution can proceed as described above. Illite and SiO2 precipitate as long as excess K+ is available in solution. The entire process of illitization, therefore, is supposed to depend on K+ availability, which is supposed to be restricted to the K+ introduced to the EBS by inflowing water (i.e. K+ availability would depend on the composition of the surrounding water). It should be noted that illitization is slower than cation-exchange reactions and is therefore probably not observable in any EBS from the beginning of its activity. However, at both the bentonite/cement and at the bentonite/iron interface the solubility of the smectite is significantly increased under high pH (cement porewater) and due to reductive dissolution (low Eh at the Fe canister), which could also lead to illitization if K+ was present.
Important information regarding illitization can be gained from natural analogue studies because the process of illitization is rather slow. In natural analogue studies in Kinnekulle, Sweden, Pusch (Reference Pusch1983) and Brusewitz (Reference Brusewitz1986) studied the influence of heat on the loss of swelling capacity due to illitization and the release of free silica by observing mineralogical changes following an intrusion into Ordovician bentonite layers. The authors demonstrated that temperatures exceeded 100°C and that the resulting heat plume influenced the bentonite layer for several hundreds of years. Pusch (Reference Pusch1983) suggested that ‘[a] possible cementation mechanism, i.e. that of quartz precipitation, is very probably associated with the smectite/illite conversion’. This was concluded due to the fact that SiO2 forms upon real illitization. The authors speculated that such silica cementation could reduce the swelling capacity of the barrier, in addition to the loss of swelling capacity caused by smectite transformation. Müller-Vonmoos et al. (Reference Müller-Vonmoos, Kahr, Bucher and Madsen1990, Reference Müller-Vonmoos, Kahr and Madsen1994) critically assessed this hypothesis; however, the authors could not distinguish between potentially large amounts of free silica from the devitrification of volcanic ash and relatively low amounts of free silica from illitization. Sauer et al. (Reference Sauer, Caporuscio, Rock, Cheshire and Jové-Colón2020) performed hydrothermal experiments with mixtures of Opalinus Clay and Wyoming bentonite up to 300°C for 6 weeks and also could not identify illitization experimentally. Cheshire et al. (Reference Cheshire, Caporuscio, Rearick, Jove-Colon and McCarney2014) studied the alteration of Wyoming bentonite in hydrothermal experiments but concluded that ‘[t]here was no evidence of illite–smectite mixed-layering from any of the experiments conducted in this investigation’. Instead, K+ was exchanged in the montmorillonite interlayer.
Several studies have attempted to quantify illitization by using semi-empirical kinetic models, with rate expressions defined either on the basis of observed mineral transformations as part of burial diagenesis or through laboratory data (e.g. Eberl & Hower, Reference Eberl and Hower1976; Pytte, Reference Pytte1982; Velde & Vasseur, Reference Velde and Vasseur1992; Huang et al., Reference Huang, Longo and Pevear1993). Karnland & Birgersson (Reference Karnland and Birgersson2006) compared two laboratory-based models proposed by Huang et al. (Reference Huang, Longo and Pevear1993) and Cuadros & Linares (Reference Cuadros and Linares1996), as well as a field-based kinetic rate expression proposed by Pytte & Reynolds (Reference Pytte A, Reynolds, Naeser and McCulloh1989), for their ability to quantify illitization as part of the KBS-3 repository concept. The model by Huang et al. (Reference Huang, Longo and Pevear1993) was regarded to be most suitable, as the applied kinetic rate expression and its associated parameters are based on laboratory work and as the model was tested independently on geological settings with reasonable agreement. The Huang et al. (Reference Huang, Longo and Pevear1993) model considers a temperature dependence of the illitization rate based on an Arrhenius type expression; it also considers the montmorillonite fraction and potassium concentration, but it does not include parameters such as pH, water content and thermal gradient (see Equation 3):
where dS/dt is the illitization rate, [K+] is the potassium ion concentration, S the smectite fraction and T the absolute temperature in Kelvin.
All models, however, agree that illitization will be relatively slow in the bentonite barrier and hence probably of less relevance compared to other reactions. Illitization has also been inferred by several numerical geochemical modelling studies. Zheng et al. (Reference Zheng, Rutqvist, Birkholzer and Liu2015, Reference Zheng, Rutqvist, Xu and Birkholzer2017) used coupled thermal, hydraulic, mechanical and chemical modelling to simulate a bentonite-backfilled EBS system in a clay formation over 1000 years and over 100,000 years. In both studies, two temperature scenarios were considered: a case in which the temperature in the bentonite near the waste canister peaked at 100°C; and another in which the temperature was allowed to reach 200°C. Illitization was simulated as a dissolution/precipitation reaction. Smectite was allowed to dissolve under the formation of illite, with the illitization rate calibrated against field data. Both studies showed illitization to occur in the bentonites as well as in the clay formation, being enhanced under higher temperatures. Illitization resulted in a notable reduction in the swelling stress of the employed bentonites. However, the model results were not verified experimentally. Approximating illitization through a dissolution/precipitation reaction was also undertaken by Savage et al. (2020), simulating the burial of marine sediments over geological timeframes and increasing temperatures. The smectite fraction within the sediment was included as a K-montmorillonite, with both illite and smectite modelled as discrete minerals rather than mixed layers. The rate and magnitude of illitization predicted by the model was verified against the observed mineralogical data, with depth determined from a site offshore of Japan. It was found that the onset of illitization of smectite with depth, time and temperature from the model was able to be matched with previous data, but the overall rate of transformation was much more rapid in the model compared to the observed mineralogical data.
Geochemical modelling of illitization requires thermodynamic data for illite/smectite and any mixed-layer minerals; however, the thermodynamic stability of illite/smectite mixed-layer minerals remains an unsolved issue due to the inherent non-stoichiometric nature of these minerals (Gailhanou et al., Reference Gailhanou, Blanc, Mikaelian, Kawaji, Olives and Amouric2012, Reference Gailhanou, Blanc, Rogez, Mikaelian, Kawaji and Olives2019). As a consequence, modelling studies generally adopt an idealized stoichiometry, which will introduce uncertainties with regards to mineral stability. The lack of a larger set of thermodynamic data representing a range of mineral compositions remains a limitation for predicting illitization rates as part of numerical long-term performance assessments of radioactive waste repository concepts.
To date, investigation of illitization remains an analytical challenge, mostly because illitization leads to the formation of mixed-layer minerals, which, from an analytical point of view, represent the most challenging of clay minerals. Important studies regarding the structural aspects of illitization, which are not discussed further in this study, have been published by Honty et al. (Reference Honty, Uhlík, Sucha, Caploviová, Francu, Clauer and Biron2004) and Meunier & Velde (Reference Meunier and Velde2004).
Mineral alteration at the bentonite/iron interface
The formation of corrosion phases at the contact with an Fe heater (or carbon steel) and the formation of secondary silicates at the cement/bentonite interface may not be considered as thermally induced reactions because they also happen at room temperature, sometimes occurring even more rapidly (Stoulil et al., Reference Stoulil, Kanok, Kouril, Parschova and Novak2013). The effect of temperature on the mechanism and/or extent of these interface reactions is rarely considered in a systematic manner. Stoulil et al. (Reference Stoulil, Kanok, Kouril, Parschova and Novak2013) reported a slightly lower corrosion rate at 90°C compared to 40°C, which the authors explained by a denser magnetite layer in cases of higher temperatures, leading to less Fe2+ diffusion. Both interface reactions (bentonite/iron and bentonite/cement) are affected but not exclusively triggered by temperature. Therefore, they are discussed briefly in the following. The large number of studies conducted on the reactions dealing with these interfaces justify a separate review. Hence, only a couple of studies are cited below.
The bentonite/copper interface is considered to be relatively inert as long as sulfides are absent. The reaction of bentonite and metallic iron (or carbon steel), however, leads to the formation of different corrosion products. Commonly, magnetite and Fe-silicates are observed. At low temperatures, 1:1 Fe-silicates (‘7 Å phases’) form, which were characterized in detail by Lanson et al. (Reference Lanson, Lantenois, van Aken, Bauer and Plançon2012). High-temperature experiments (i.e. >150°C) often resulted in the formation of chlorites (‘14 Å phases’; Guillaume et al., Reference Guillaume, Neaman, Cathelineau, Mosser-Ruck, Peiffert and Abdelmoula2003; Wersin et al., Reference Wersin, Jenni and Maeder2013; Zandanel et al., Reference Zandanel, Sauer, Rock, Caporuscio, Telfeyan and Matteo2022), sometimes accompanied by zeolites and even Fe-smectite. Apart from the identification and characterization of corrosion products, the reaction mechanism has also been investigated (Kaufhold et al., Reference Kaufhold, Klimke, Schlömer, Alpermann, Renz and Dohrmann2020b). Firstly, Fe corrosion leads to the formation of hydrogen, which in turn reduces structural ferric iron in the smectites (both at room temperature or elevated temperatures). Based on this reaction, the bentonite has to be understood as an Eh buffer. Indeed, Gorski et al. (Reference Gorski, Klüpfel, Voegelin, Sander and Hofstetter2013) showed a correlation between Eh and layer charge density, and Kaufhold et al. (2015) were able to show a correlation between corrosion rate and layer charge density. Gorski et al. (Reference Gorski, Klüpfel, Voegelin, Sander and Hofstetter2013) argue that these correlations are a consequence of low-charge smectites being more oxidizing than high-charged smectite, which, in turn, explains the higher corrosion rates of low-charge smectites (or bentonites containing low-charge smectites). The different extents of corrosion at the Fe interface of different bentonites can be understood based on the reducibility of structural Fe3+. In other words, different smectites require different H2 concentrations at which significant Fe3+ reduction starts. Quantifying corrosion rates within a numerical model would therefore require an understanding of the Eh buffering capacity of different smectites. As a result of the reduction of structural Fe3+, smectites become less stable and hence more soluble. This increased solubility is referred to as ‘reductive dissolution’ (Jaisi et al., Reference Jaisi, Kukkadapu, Eberl and Dong2005). It leads to the formation of Fe-silicates, but the presence of carbonates may additionally and/or alternatively lead to the formation of Fe-carbonates. The rate of formation of Fe-silicates should, therefore, be faster than expected based on regular smectite dissolution rates.
Consistent results were reported on the corrosion rate ranging from 1 to 5 μm year–1 (Foct & Gras, Reference Foct, Gras, Ferron and McDonald2003; Bildstein et al., Reference Bildstein, Trotignon, Perronnet and Jullien2006; Savage et al., Reference Savage, Watson, Benbow and Wilson2010; Stoulil et al., Reference Stoulil, Kanok, Kouril, Parschova and Novak2013; Smart et al., Reference Smart, Reddy, Rance, Nixon, Frutschi, Bernier-Latmani and Diomidis2017; Chaparro et al., Reference Chaparro, Finck, Metz and Geckeis2021). For empirical modelling, it is therefore feasible to use an average value or maximum and minimum values. A number of modelling studies have been undertaken on iron–bentonite interactions, with most studies assuming fully anoxic conditions over the duration of the model time, disregarding the initial period of oxygen consumption given its short duration (e.g. Marty et al., Reference Marty, Fritz, Clément and Michau2010). Accordingly, corrosion of iron/steel containers is generally simulated as an anaerobic reaction, assuming that sufficient water is available to maintain a continuous redox reaction. Frequently, corrosion rates are simulated as constant over time (1–5 μm year–1). Some authors, however, have introduced kinetic corrosion rates. Samper et al. (Reference Samper, Naves, Montenegro and Mon2016), who simulated the interactions of corrosion products and compacted bentonite over 1 million years, took the thermal gradient across the bentonite buffer into account, as well as the temperature dependence of the corrosion rate. Their results suggest that the initial corrosion rate increases from 2.00 to 4.75 μm year–1 and then decreases after 10,000 years to ~2.2 μm year–1. The authors found that the temperature dependence of the corrosion rate did not result in any significant influence on the geochemical evolution of the bentonite buffer over the longer term. Others employed kinetic corrosion rates with a rate dependence on the dissolved Fe2+ concentration (e.g. Marty et al., Reference Marty, Fritz, Clément and Michau2010; Lu et al., Reference Lu, Samper, Fritz, Clement and Montenegro2011). It is noteworthy that this led to a significant reduction in the maximum corrosion rate of the simulated canisters to <1 μm year–1. Similarly, Wilson et al. (Reference Wilson, Benbow, Sasamoto, Savage and Watson2015) simulated kinetic corrosion with the rate limited by the diffusive supply of hydrogen to the iron/steel surface. This also led to a significant reduction in anaerobic steel corrosion (0.06 μm year–1) compared to the static corrosion rates.
The production of hydrogen gas as part of canister corrosion and its implications for the integrity of the bentonite barrier are not well understood at present, and, accordingly, modelling approaches divert. Although Xu et al. (Reference Xu, Senger and Finsterle2008) explicitly simulated H2 gas generation and the associated pressure build-up, showing that gas pressure build-up was strongly correlated with the assumed corrosion rate, many modelling studies do not consider vapour phases and simulate the bentonite barrier as a fully water-saturated system. In these models, corrosion products such as Fe2+ are released along with hydrogen, which migrate through the clay barrier by diffusion (Montes et al., Reference Montes, Marty, Fritz, Clement and Michau2005; Samper et al., Reference Samper, Lu and Montenegro2008; Marty et al., Reference Marty, Fritz, Clément and Michau2010; Ngo et al., Reference Ngo, Delalande, Clément, Michau and Fritz2014; Wilson et al., Reference Wilson, Benbow, Sasamoto, Savage and Watson2015). In essence, these models simulate the reaction of bentonite with corrosion product-equilibrated water rather than simulating a physical contact between bentonite and a steel canister.
The transformations of primary bentonite minerals in the near field of a spent-fuel Fe/steel canister are simulated to produce Fe2+-rich alteration products. However, model assumptions such as the secondary minerals included in the model, the kinetic rate laws employed, the reactive surface areas assumed, the thermodynamic database used and whether isothermal conditions or the evolution of the temperature is considered result in variations in the simulated bentonite alterations. Wilson et al. (Reference Wilson, Benbow, Sasamoto, Savage and Watson2015) simulated Fe2+-rich clays as the products of direct contact between bentonite and the steel canister. Similarly, Chaparro et al. (Reference Chaparro, Finck, Metz and Geckeis2021) predicted Fe-silicate phases (greenalite, nontronite) as corrosion products together with magnetite. Others have simulated magnetite and siderite as the main steel corrosion products at the interface with compacted bentonite (Samper et al., Reference Samper, Lu and Montenegro2008; Lu et al., Reference Lu, Samper, Fritz, Clement and Montenegro2011; Samper et al., Reference Samper, Naves, Montenegro and Mon2016), aluminosilicates such as Fe–Al-chlorite and Mg–Al-chlorite (Marty et al., Reference Marty, Fritz, Clément and Michau2010; Ngo et al., Reference Ngo, Delalande, Clément, Michau and Fritz2014) and serpentine-like minerals such as berthierine, cronstedtite and chamosite (Bildstein et al., Reference Bildstein, Trotignon, Perronnet and Jullien2006). Savage et al. (Reference Savage, Watson, Benbow and Wilson2010) concluded that more data on Fe-silicates are needed to improve Fe-bentonite modelling. The effect of layer charge density on the reducibility and hence solubility of smectites explained above has not yet been included in geochemical modelling of bentonite/iron interactions, which would explain why Fe-silicates are hardly predicted by modelling (as in the NF-PRO project; Arcos et al., Reference Arcos, Cuevas, Fernández, Herbert, Hernan and Van Loon2008).
In summary, different corrosion products were identified at the iron/bentonite interface. Most commonly, magnetite is sometimes found directly at the heater (not necessarily), followed by a Fe-silicate layer (7 Å or 14 Å depending on the temperature). The corrosion rate was consistently reported to range from 1 to 5 μm year–1. The entire system is not yet fully understood in terms of the effects of different bentonite components (opals, carbonates, interlayer compositions, etc.) on the corrosion rate and the formation of different corrosion products at different temperatures. Systematic studies are lacking, which should be accompanied by modelling to improve the available thermodynamic data, particularly those regarding the potential Fe-rich siliceous corrosion products listed above.
Mineral alteration at the bentonite/cement interface
As explained in the previous section, neither reaction – iron/bentonite nor cement/bentonite – is thermally induced. Nevertheless, a large number of publications are available on both reactions due to their relevance for the integrity of EBS systems and due to their complexity. A recent and comprehensive summary of studies on the bentonite/cement interaction was published by Wieland et al. (Reference Wieland, Mäder, Lothenbach, Jenni and Bernard2017). In the present paper, therefore, only brief summaries of the reaction and approaches to modelling it are provided.
The cement/bentonite interface may not exist in all EBS concepts, but the topic was and is investigated extensively (Oda et al., Reference Oda, Honda, Savage, Metcalfe and Walker2004; Gaucher et al., Reference Gaucher and Blanc2006; Savage et al., Reference Savage, Walker, Arthur, Rochelle, Oda and Takase2007). In addition, the temperature may be lower at the bentonite/cement interface compared with the canister/bentonite interface, as cement plugs (or similar), which are used to seal shafts, will be farther away from the canisters. The cement porewater of ordinary Portland cement is known to be alkaline due to hydrolysis, mainly of Ca-oxide. The alkaline solutions significantly facilitate the dissolution of smectites, and, depending on the chemical composition of the smectite and possible minor phases, different alteration products form (e.g. Yokoyama et al., Reference Yokoyama, Shimbashi, Minato, Watanabe, Jenni and Mäder2021). Sawaguchi et al. (Reference Sawaguchi, Tsukada, Yamaguchi and Mukai2016) reported on high-pH alteration experiments with Kunipia-F bentonite, with a focus on dissolution of montmorillonite at elevated temperatures (50–90°C) in relation to the activity of OH–. Differences in dissolution rates of montmorillonite were explained by considering OH– activity accompanied by the dissolution of accessory minerals such as quartz and chalcedony. Recent summaries of both experimental and modelling approaches have been provided by Wieland et al. (Reference Wieland, Mäder, Lothenbach, Jenni and Bernard2017) and Jacques et al. (Reference Jacques, Phung, Perko, Seetharam, Maes and Liu2021). Kaufhold et al. (Reference Kaufhold, Dohrmann and Ufer2020a) investigated the cement/bentonite interface based on mixing powders, which allowed for the application of bulk analysis as a representative of the interfaces. They showed that the degree to which smectite is dissolved depends on the amount of reactive silica that is present in bentonites (e.g. as opal-A). The experimental data confirmed previous modelling of this buffered reaction by Arthur & Savage (Reference Arthur and Savage2016). The thermodynamic data regarding the possible alteration products such as sulphates, carbonates, feldspar and zeolites are considered to be more accurate than those for smectite. Successful modelling of these reactions may also result from the fact that smectites dissolve congruently at high pH, which is more straightforward to simulate than incongruent dissolution.
Systematic data regarding temperature effects concerning the cement/bentonite reaction are available (Fig. 8). Kaufhold et al. (Reference Kaufhold, Dohrmann and Ufer2020a) varied the temperature of bentonite/cement/water mixtures from 30°C to 80°C (although the temperature at the bentonite/cement interface in a real repository may be well below 80°C because of its larger distance from the canister). The results were evaluated based on a comparison of CEC data at different temperatures, which were shown to correspond to the extent of smectite degradation. Some bentonites showed significant increases in the extent of smectite degradation between 30°C and 60°C (e.g. increase from 45% to 80%), but less so between 60°C and 80°C (+5%), whereas other bentonite blocks showed an almost linear slope.

Figure 8. Extent to which smectites are dissolved in contact with cement depending on the temperature (Kaufhold et al., Reference Kaufhold, Dohrmann and Ufer2020a).
In summary, the reaction of bentonite with cement is driven by the dissolution of smectite and the precipitation of secondary phases, with the type depending on the chemical composition. Because of the high pH, smectite dissolution is presumably congruent, which is advantageous with respect to numerical modelling.
Conclusions
Many of the mineralogy-related reactions observed in bentonite barrier systems were summarized and discussed, highlighting long-term, large-scale and laboratory experiments. As the relevant phenomena are essentially all influenced by temperature and most large-scale experiments are conducted at elevated temperatures, a clear emphasis was placed on the temperature dependence of the respective phenomena.
The long-term safety assessment of barrier systems has to rely on numerical modelling. To arrive at realistic model predictions, all relevant reactions need to be identified and reasonable input parameters have to be established. Given the complexity of a barrier system and the multitude of phenomena that occur in such a setting, this is certainly a stepwise process.
From the discussion of the dissolution of the main bentonite components, it appears that the (temperature-dependent) dissolution of smectites deserves more attention. This is especially evident when considering the increase in magnesium concentrations close to heaters, which could be ascribed to incongruent dissolution. Considering the dissolution of smectites at bentonite/cement interfaces, the situation might be less complicated because, due to comparatively high pH values, the smectite could be assumed to dissolve congruently. A further difficulty can be expected at the interface of bentonite and iron/steel, where, as a result of the reduction of structural iron, reductive dissolution of smectites may occur. These processes, together with ion-exchange equilibria at elevated temperatures driven by changes in groundwater composition, can be expected to impact the long-term safety assessment of barrier systems and deserve further attention.
Acknowledgements
The present review was motivated by the HotBent Team (project head: Dr Florian Kober), who requested of us to summarize the challenges of modelling thermally induced reactions in bentonite buffers.











