No CrossRef data available.
Published online by Cambridge University Press: 14 March 2018
The hydroxides of the divalent metals have an octahedrally coordinated structure of hexagonal symmetry typified by the “cadmium hydroxide” structure, familiar to clay mineralogists as the “brucite” structure. The weakly electropositive metals form insoluble or slightly soluble basic salts in which the ratio 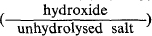 increases as the initial concentration of the unhydrolysed salt is decreased. A pseudo-Cd(OH)2 structure is formed when this ratio is of the order of 20 to 25 in which the octahedral sheets are separated by a constant distance proportional to the radius of the anion of the unhydrolysed salt. The resultant characteristic interplanar spacing is stabilised by a very small number of anions, the remaining interlamellar space being filled with amorphous hydroxide. The latter is known as the “α -zinc hydroxide” structure since zinc shows its similarity to its fellow transitional group metals by forming basic salts with this structure (Feitknecht, 1938).
increases as the initial concentration of the unhydrolysed salt is decreased. A pseudo-Cd(OH)2 structure is formed when this ratio is of the order of 20 to 25 in which the octahedral sheets are separated by a constant distance proportional to the radius of the anion of the unhydrolysed salt. The resultant characteristic interplanar spacing is stabilised by a very small number of anions, the remaining interlamellar space being filled with amorphous hydroxide. The latter is known as the “α -zinc hydroxide” structure since zinc shows its similarity to its fellow transitional group metals by forming basic salts with this structure (Feitknecht, 1938).