Case report
An 8-year-old boy (28 kg), initially diagnosed with a hypoplastic aortic arch and coarctation, was referred to us with significant restenosis in the left ventricular outflow tract (echocardiographic peak gradient – 80 mmHg) and a stent in the aortic isthmus (39 mmHg), (Fig. 1). His previous treatment included neonatal surgical correction of the aortic arch and isthmus, followed by an early re-coarctation stenting and subsequent aortic arch reoperation with partial stent resection at the age of 1 year, as well as subaortic stenosis resection at the age of 3 years.
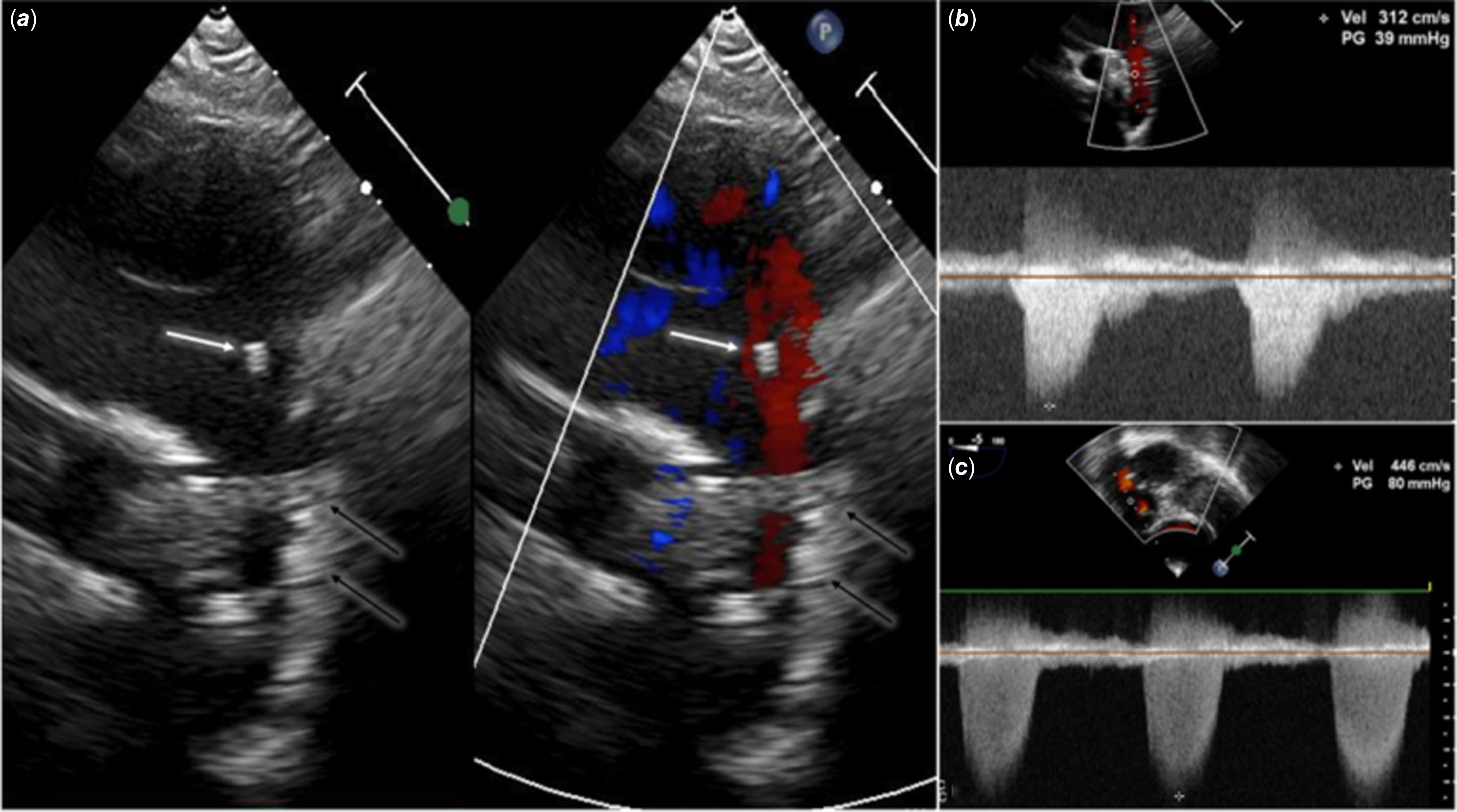
Figure 1. Echocardiography in a patient after multiple surgical and percutaneous interventions for hypoplastic aortic arch and left ventricular outflow tract obstruction. a: Transthoracic echocardiography, suprasternal long-axis view of the aortic arch: an additional “structure” (white arrow) was initially taken for an artefact, and attention was focused on the previously implanted stent (black arrows). b: Transthoracic echocardiography: continuous wave Doppler shows increased velocity through the stent in the aortic isthmus (39 mmHg). c: Transoesophageal echocardiography, trans-gastric projection: left ventricular outflow tract obstruction – peak gradient 80 mmHg.
Cardiac catheterisation was performed to accurately delineate levels of obstruction and potentially redilate the stent. Haemodynamic measurements showed significant systolic gradient across the outflow tract (67 mmHg), with a trivial gradient across the stent in the isthmus (3 mmHg). Three-dimensional rotational angiography revealed pseudoaneurysms at the previously implanted stent region and the presence of an additional linear structure dividing the aortic arch into two sections, not clearly visible in a two-dimensional angiography (Fig. 2, Supplementary video 1). Subsequent contrast-enhanced CT did not provide a clear explanation (Fig. 3, Supplementary video 2). A virtual reality model (VMersive) created from the CT scan enabled detailed imaging of the aortic arch and its surroundings (Fig. 4, Supplementary video 2). Following advanced imaging analysis, the patient was qualified for hybrid treatment, including resection of the left ventricular outflow tract obstruction and a covered stent implantation in the isthmus area. This case illustrates superiority of virtual modelling over standard non-invasive and advanced three-dimensional invasive imaging for complete visualisation of complex aortic arch anatomy. Reference Szeliga, Kołcz, Piwowarczyk and Góreczny1
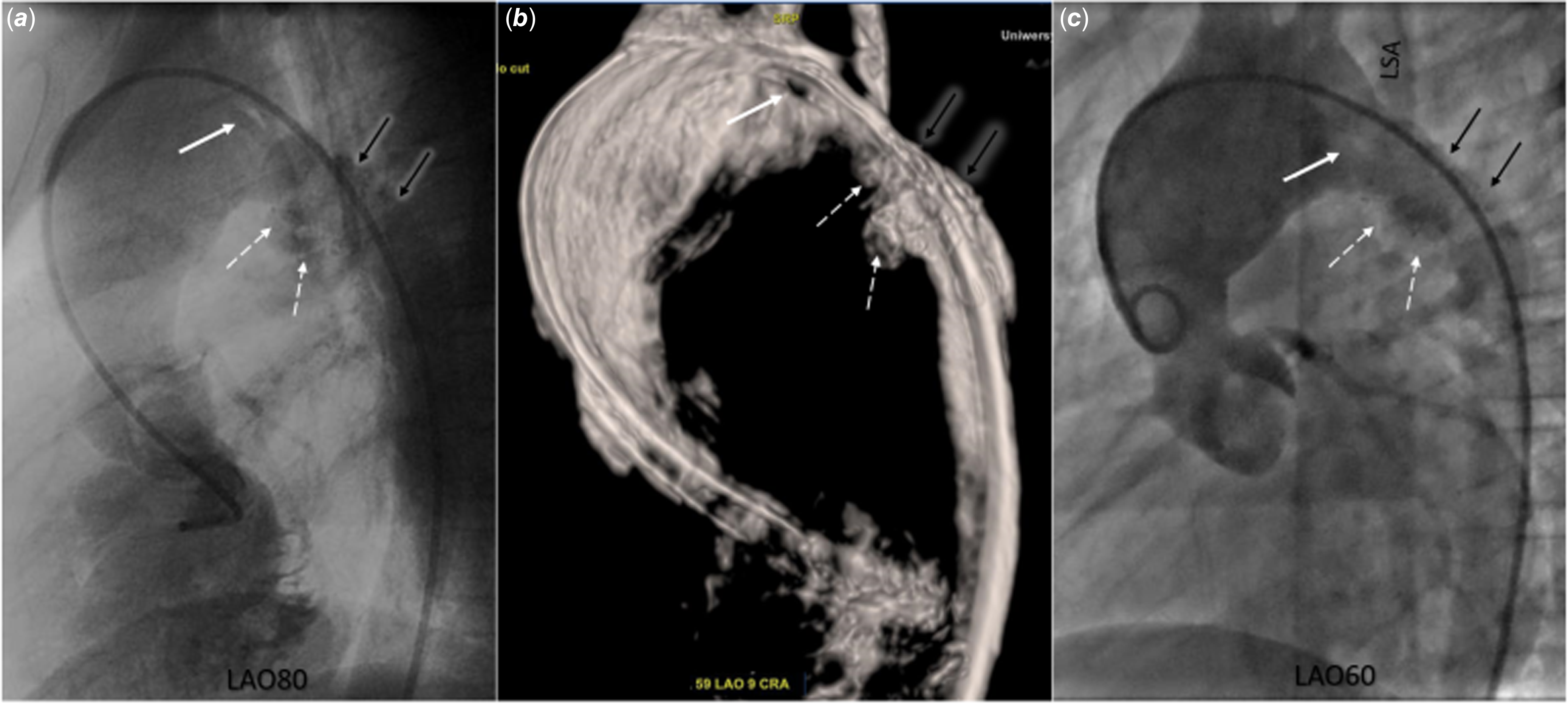
Figure 2. Three-dimensional rotational and standard two-dimensional angiography in a patient after multiple surgical and percutaneous interventions for hypoplastic aortic arch and left ventricular outflow tract obstruction. a: Rotational angiography: an additional “structure” dividing the aortic arch into two segments (white arrow); and pseudoaneurysms (white dashed arrows) in the region of the previously implanted stent (black arrows). b: Three-dimensional reconstruction from rotational angiography: an additional “structure” dividing the aortic arch into two segments (white arrow), and pseudoaneurysms (white dashed arrows) in the region of the previously implanted stent (black arrows). c: Two-dimensional angiography: poorly visible negative contrast indicating the presence of an additional “structure” dividing the aortic arch into two segments (white arrow); and pseudoaneurysms (white dashed arrows) in the region of the previously implanted stent (black arrows). LSA = left subclavian artery.
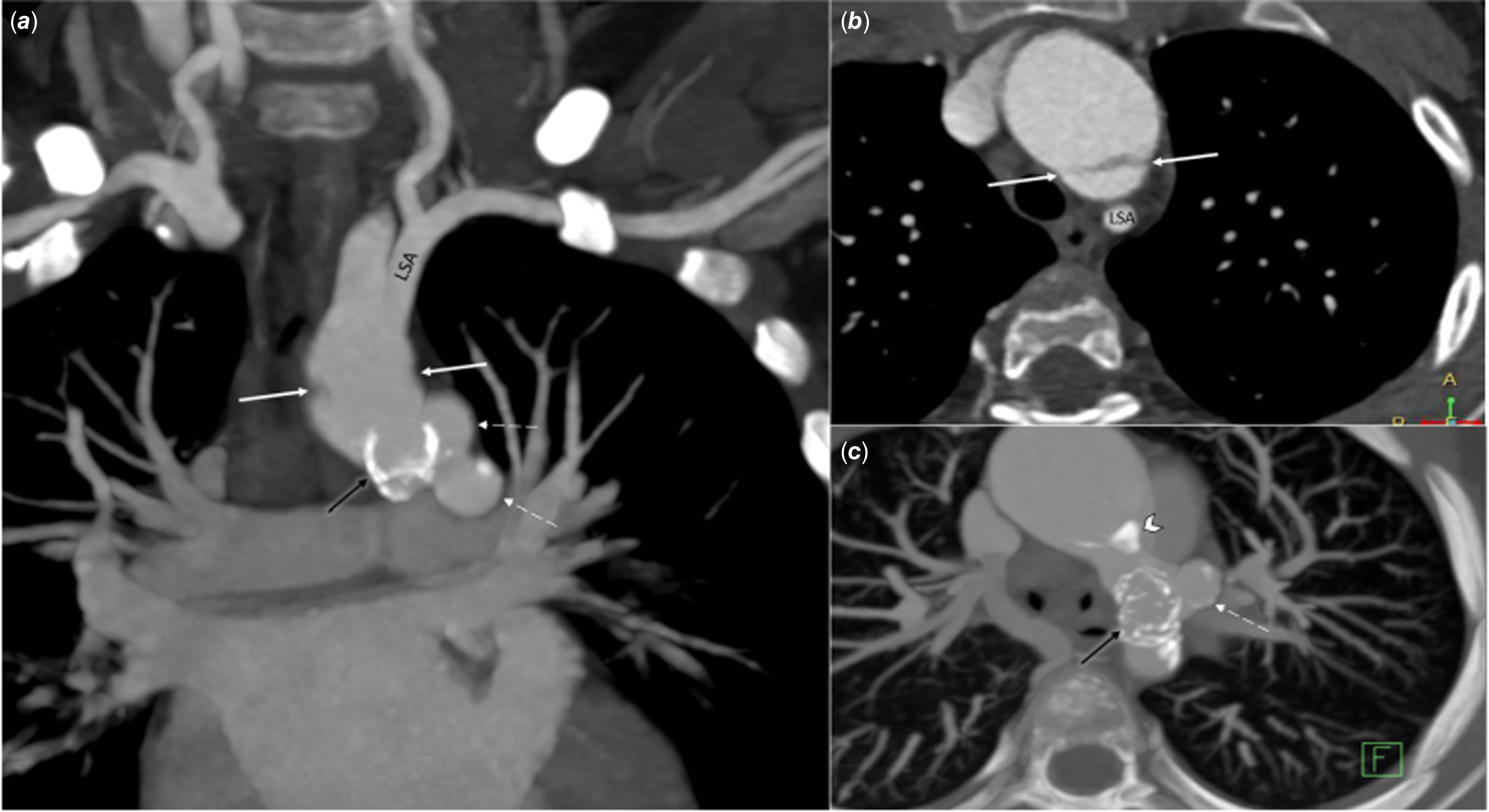
Figure 3. Contrast-enhanced CT in a patient after multiple surgical and percutaneous interventions for hypoplastic aortic arch and left ventricular outflow tract obstruction. a: Cranial projection: negative contrast on both sides of the transverse arch (white arrow); pseudoaneurysms (white dashed arrows) in the region of the previously implanted stent (black arrows). b: Transverse projection: linear negative contrast in the transverse arch similar to aortic dissection (white arrow). c: Transverse projection: pseudoaneurysms (white dashed arrow) at the level of the stent (black arrow), calcifications in the distal part of the aortic arch (white arrow head); LSA = left subclavian artery.
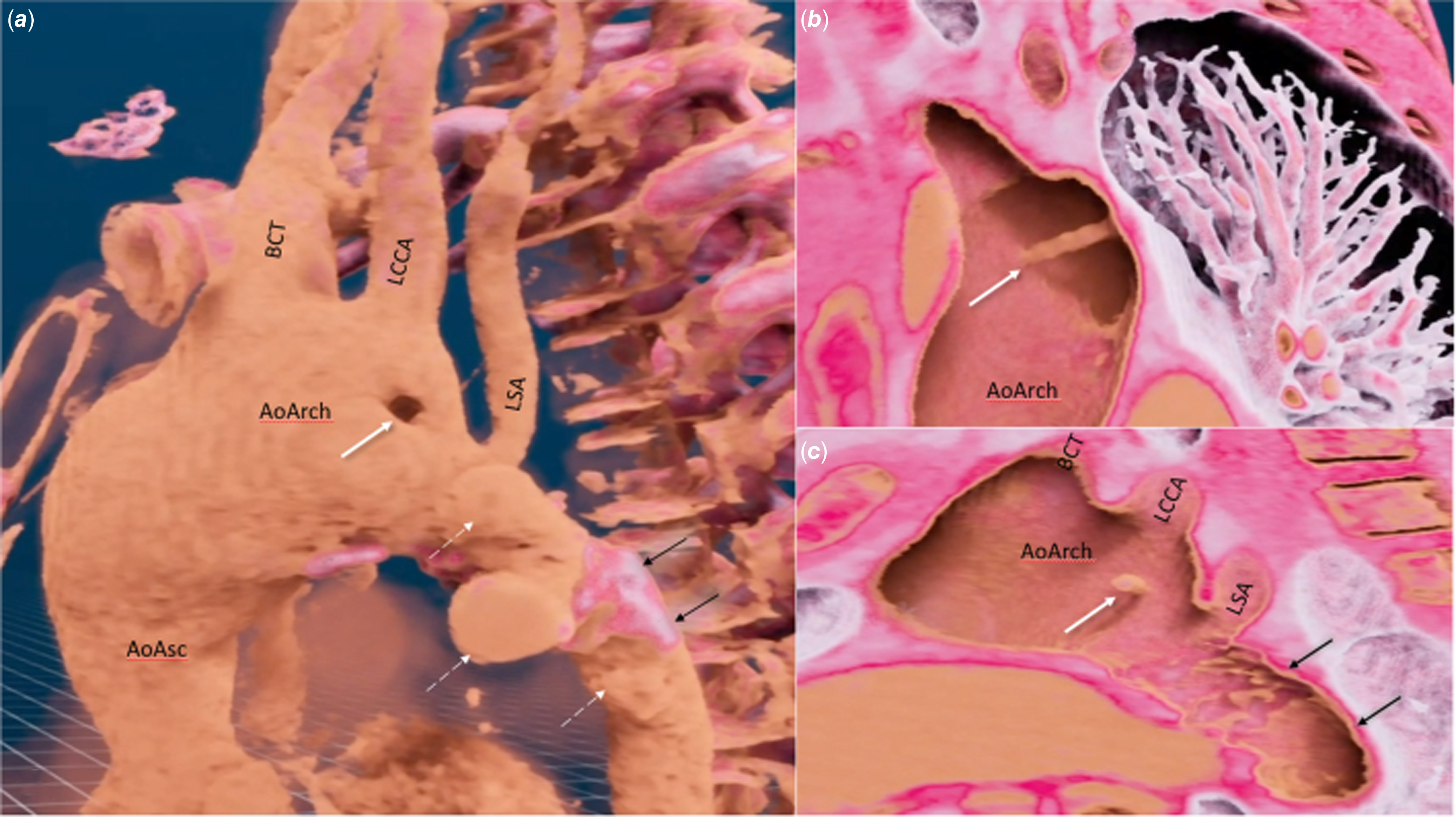
Figure 4. Virtual reality model (VMersive) based on CT scan in a patient after multiple surgical and percutaneous interventions for hypoplastic aortic arch and left ventricular outflow tract obstruction. a: Linear structure (white arrow) dividing transverse aortic arch into two segments; and pseudoaneurysms (white dashed arrows) in the region of the previously implanted stent (black arrows). b: Linear structure dividing the aortic arch into two lumens (white arrow). c: Three-dimensional virtual model in a lateral projection presenting the linear structure (white arrow) and its relations to aortic arch branches and the previously implanted stent (black arrows). AoAsc = ascending aorta, AoArch = aortic arch, BCT = brachiocephalic trunk, LCCA = left common carotid artery, LSA = left subclavian artery.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S1047951124000386.
Acknowledgements
To Anna Grondalski from Pomeranian Medical University for editing the text.
Financial support
Virtual Reality project is supported by the Jagiellonian University Medical College internal grant No. N41/DBS/001219.
Competing interests
None.







