Case report
This baby was born at 39 weeks gestation with an antenatal diagnosis of pulmonary atresia with intact ventricular septum. At birth, prostaglandin E1 was started and the baby was transferred to our institution for establishment of definitive pulmonary blood flow.
On arrival, the arterial oxygen saturation was 90%. Echocardiography confirmed the antenatal diagnosis and suggested favourable anatomy for biventricular repair with a bipartite right ventricle and a good-sized tricuspid valve (1.1 cm, z-score −0.4). There was membranous pulmonary atresia with a pulmonary annular dimension of 0.67 cm (z-score −1.3). The estimated right ventricular systolic pressure was 112 mmHg via continuous wave Doppler assessment of the severe tricuspid regurgitation. Based on this information, the baby was taken to the catheterisation laboratory for wire perforation of the pulmonary valve to allow antegrade flow. The wire was unable to be advanced across the valve, and therefore the arterial duct was stented to secure pulmonary blood flow. The baby tolerated the procedure well.
Within hours of the procedure, the baby started to have episodes of desaturation and respiratory distress. Stabilisation was seemingly achieved after sedation and intubation, but the baby went on to have profound and persistent desaturations to approximately 30%. During these desaturation events, the mean arterial pressure also dropped by approximately 15 mmHg.
A chest X-ray showed oligaemic lung fields when compared to the film immediately following stent insertion (Fig. 1). Echocardiography showed the ductal stent was patent with laminar flow. To increase arterial saturation and promote pulmonary blood flow, the intensive care team administered a red blood cell transfusion, inhaled nitric oxide, and norepinephrine and epinephrine to raise blood pressure and increase left-to-right ductal flow. These interventions were unsuccessful in resolving the episodic desaturation. A pattern emerged during this resuscitation whereby intravenous fluid boluses consistently had the transient effect of increasing the oxygen saturation and blood pressure for 15–30 minutes at a time. An echocardiogram obtained during resuscitation showed that the hypertensive right ventricle was compressing the left ventricle (Fig. 2). The team concluded that right ventricular decompression would be advantageous in promoting both cardiac output and pulmonary blood flow.

Figure 1. (a) Normal pulmonary vascularity immediately after ductal stent insertion. (b) Oligaemic lung fields during period of severe oxygen desaturation.
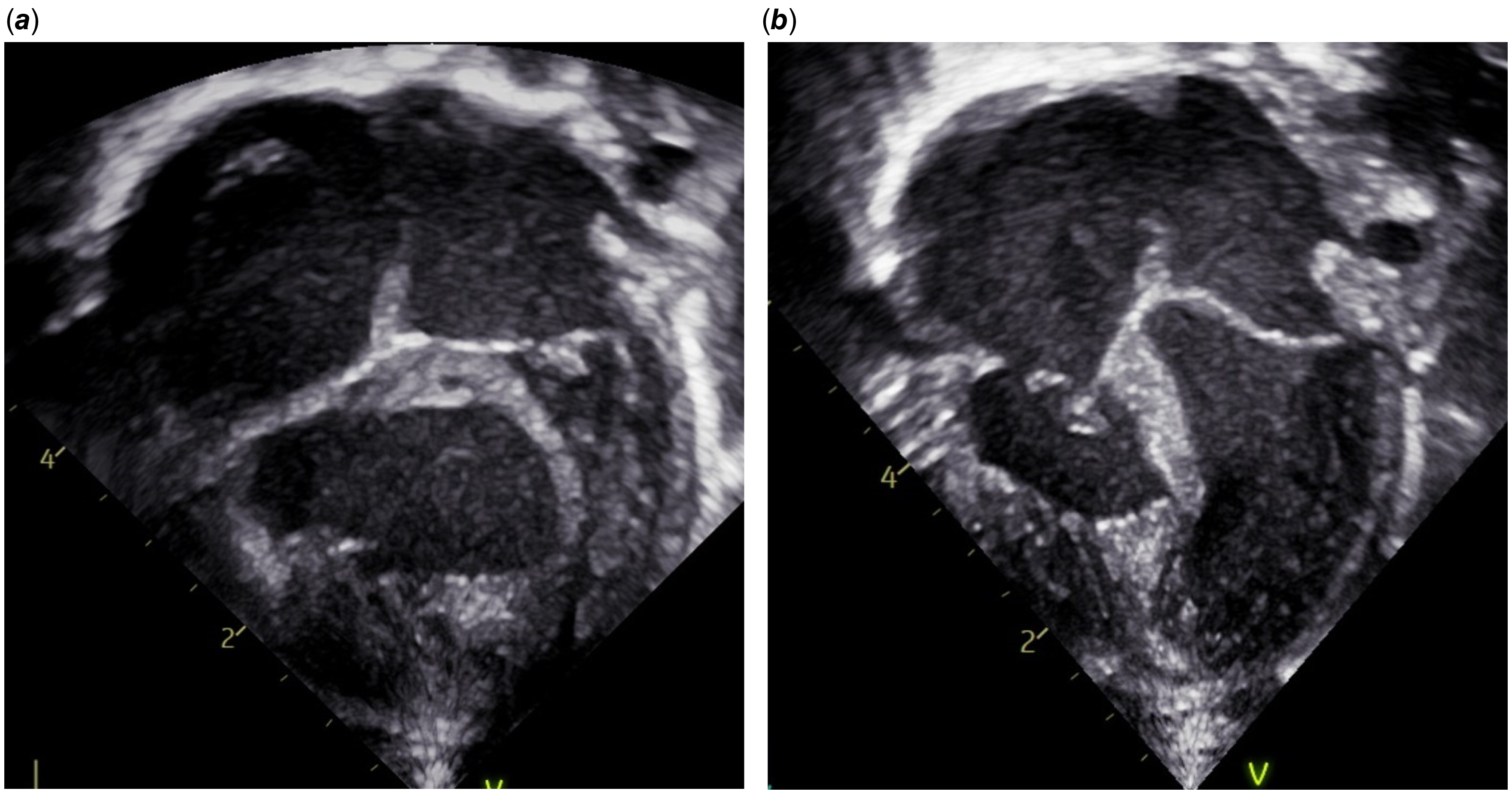
Figure 2. (a) Apical four chamber view showing compression of the left ventricle by the hypertensive right ventricle . (b) Apical four chamber view after right ventricle decompression.
The baby was brought to the operating room for surgical decompression of the right ventricle with a small transannular patch from bovine pericardium. The ductal stent was removed, and the patent ductus arteriosus was ligated and divided. A 3 mm Blalock-Taussig-Thomas shunt was placed and an atrial septectomy was performed.
Review of the post-surgical echocardiogram showed improvement in right ventricular hypertension and septal bowing (Fig. 2), and an unobstructed right ventricular outflow tract. There was only mild tricuspid regurgitation with an estimated right ventricular pressure of 48 mmHg, which was 62 per cent of the systemic pressure. Oxygen saturations at the end of the case were 88–92%.
Discussion
Ventricular-ventricular interactions describe the interdependence of right and left ventricular systolic and diastolic functions. The physiologic basis for these interactions is related to a common pericardium, shared myofibers, and the position of the interventricular septum. Reference Friedberg1
This patient had a dilated, hypertensive right ventricle. The combination of high central venous pressure driving third space losses of intravascular volume and shift of the interventricular septum to the left led to progressive loss of left ventricular preload. Decreased cardiac output combined with high left atrial pressure reduced blood flow through the stented ductus and resulted in profound cyanosis. Administration of fluid temporarily reversed the process by improving left ventricular filling and cardiac output, but this could not be maintained because, over time, the process repeated itself as intravascular volume decreased due to the persistently unfavourable hydrostatic forces imposed by the lack of right ventricular decompression.
Haemodynamic instability has been previously described in a patient with pulmonary atresia, intact ventricular septum, and large hypertensive right ventricle, requiring urgent decompression. Reference Amin, Levi, Likes and Laks2 However, that patient was noted to have left ventricular outflow tract obstruction, which was not present in this case.
After the initial palliation, the baby had a stable course with gradual improvement in saturation. At nine months of age, she had a biventricular repair consisting of a monocusp patch repair of the right ventricular outflow tract with a 16 mm Contegra valved conduit, Blalock-Taussig-Thomas shunt take-down, right ventricular muscle bundle resection, tricuspid valve repair, and atrial septation with fenestrated patch. At last follow-up, she had saturations in the high 90’s, though her atrial communication continued to shunt right-to-left as a result of restrictive RV physiology.
In cases of pulmonary atresia with intact ventricular septum and a well-developed right ventricle, the hypertensive right ventricle is usually amenable to early decompression in the catheter laboratory. Reference Marasini, Gorrieri and Tuo3,Reference Cao, Tian and Rychik4 In this case, the right ventricle was not able to be decompressed, and over time the distension affected left ventricular filling. This case highlights the importance of using echocardiography images to construct potential physiological explanations for bedside phenomena. Morphology and ventricular-ventricular interactions may be overlooked when attempting to troubleshoot complex physiology but recognition of these types of interactions can be the key to finding successful therapeutic options.
Financial support
None.
Competing interests
None.





