Primary cardiac tumours in the paediatric population are rare with cardiac rhabdomyomas representing the most common histologic observations. Reference Tzani, Doulamis, Mylonas, Avgerinos and Nasioudis1 Rhabdomyomas are closely associated with tuberous sclerosis complex, a multisystem neurocutaneous genetic disorder, as 96% of infants with cardiac rhabdomyomas will ultimately be diagnosed with tuberous sclerosis complex. Reference Tzani, Doulamis, Mylonas, Avgerinos and Nasioudis1–Reference Curatolo, Bombardieri and Jozwiak3 This condition has an incidence between 1 in 6,000 and 1 in 10,000 live births, with a variable presentation involving mostly benign and non-invasive circumscribed lesions in the heart, brain, skin, kidneys, eyes, and lungs. Reference Northrup and Krueger2–Reference Northrup, Aronow and Bebin4 The diagnostic criteria for tuberous sclerosis complex have been updated in 2012, classifying into clinical and genetic diagnostic criteria; the clinical criteria are further subcategorised into major and minor features. Reference Northrup and Krueger2 As a result, the definite diagnosis of tuberous sclerosis complex can be made upon detection of pathogenic mutations of the genes tuberous sclerosis complex 1 and tuberous sclerosis complex 2, regardless of the clinical findings. Reference Northrup and Krueger2,Reference Northrup, Aronow and Bebin4 Cardiac rhabdomyomas are major features, resulting in the possibility of antenatal diagnosis when observed along with subependymal nodules on fetal ultrasound and MRI. Reference Northrup and Krueger2,Reference Curatolo, Bombardieri and Jozwiak3 Postnatally, the clinical presentation of cardiac rhabdomyomas varies from incidental discovery to signs of cardiovascular haemodynamic effects, such as cardiac tamponade, obstruction, or systemic embolisation. Reference Poterucha, Kochav, O’Connor and Rosner5 Furthermore, the association of arrhythmias with cardiac rhabdomyomas is well established encompassing supraventricular tachycardia that includes atrial flutter, atrial fibrillation, Wolff-Parkinson-White syndrome, heart block, and even ventricular tachyarrhythmias. Reference Northrup and Krueger2,Reference Curatolo, Bombardieri and Jozwiak3 We present a case of paediatric tuberous sclerosis complex with multiple cardiac rhabdomyomas, which exhibited recurrent supraventricular tachycardia non-responsive to first-line treatment.
Case report
A female neonate, delivered at 40 weeks and 1 day gestational age with Apgar scores 4, 8, and 9 at 1, 5, and 10 minutes respectively (birth weight 3.4 kg), was admitted to the neonatal ICU due to temperature instability (hypothermia), persistent hypoglycaemia (point of care glucose ranging from 38 to 44 mg/dl), and hypothyroidism (thyroid-stimulating hormone 597 μIU/mL [reference range: 0.70–5.97 μIU/mL] and free thyroxine 4 of 0.55 ng/dL [reference range: 0.85–1.75 ng/dL]). Following the presentation, thyroxine therapy at a dose of 37.5 mcg/day was started. In the neonatal ICU, at age 3 days, the baby was noted to be tachycardic on the cardiac monitor with a heart rate of 250 bpm. While the baby was calm appearing, an electrocardiogram demonstrated a rapid, narrow-complex tachyarrhythmia determined to be supraventricular tachycardia. Vagal manoeuvre (ice pack to the face) converted the rhythm to sinus, supporting a reentrant circuit, but the episode recurred a few hours later (Fig. 1a). Subsequent vagal manoeuvres were unsuccessful necessitating pharmacologic cardioversion with intravenous adenosine which terminated the supraventricular tachycardia to a non-preexcited sinus rhythm (Fig. 1b). The patient was subsequently started on 1 mg/kg/day propranolol. After the patient stabilised, an echocardiogram revealed multiple echogenic homogeneous and hyperechoic nodular masses embedded in the left ventricular and right ventricular myocardium Figure 2a–d. These nodules did not affect valves or cause any inflow or outflow obstruction on both 2D and Doppler echocardiography. Further assessment showed normal biventricular systolic function. Following the observation of these nodules, the provisional diagnosis was cardiac rhabdomyomas related to tuberous sclerosis (possible tuberous sclerosis complex diagnosis [one major feature], Table 1). A detailed clinical dermatologic physical examination showed no skin lesions consistent with tuberous sclerosis complex.
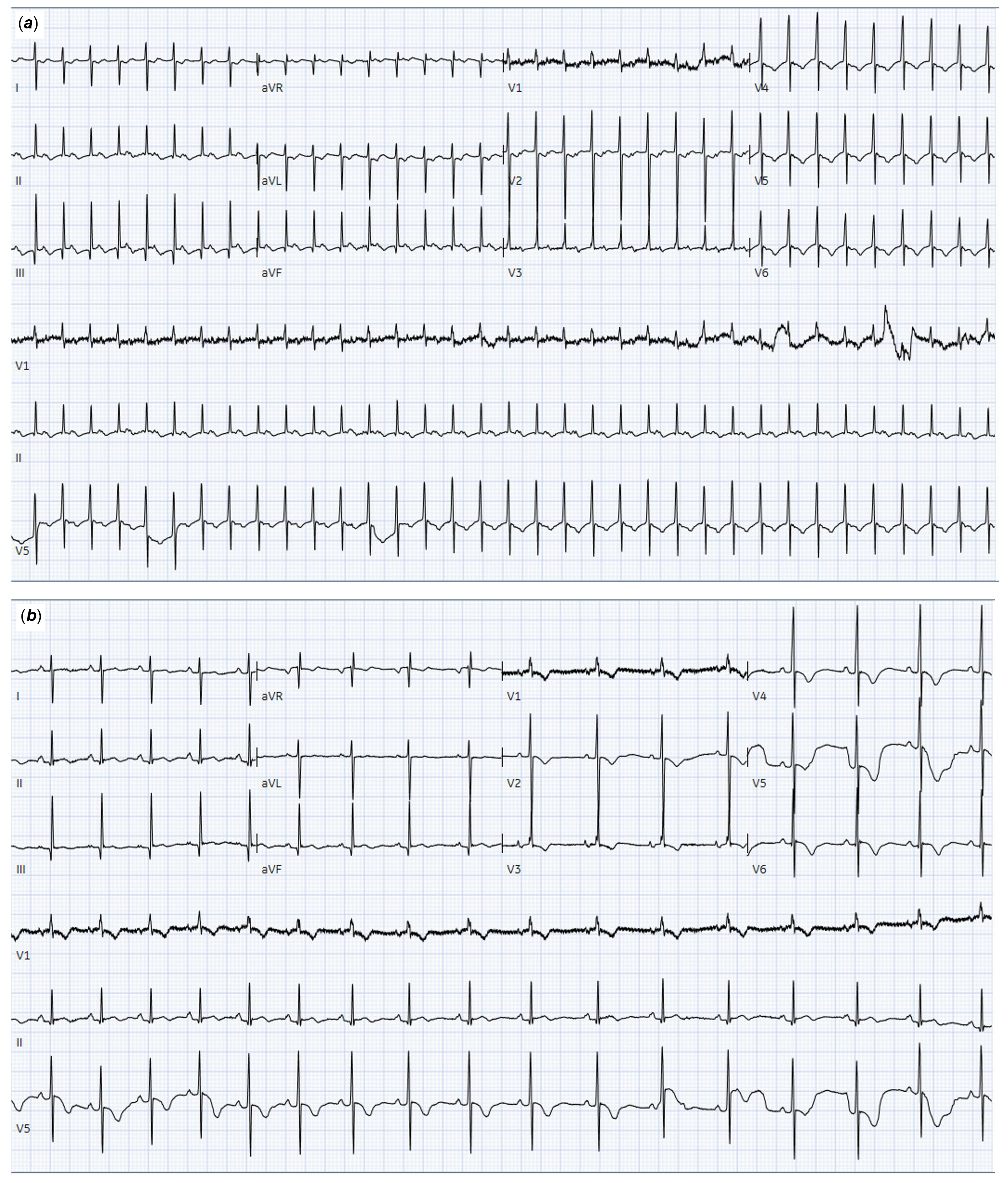
Figure 1. Patient ECG at age 3 days (a) and after cardioversion of SVT to sinus rhythm by adenosine infusion (b). ECG reveals a regular narrow-complex tachycardia at 211 bpm, inverted T-waves in lateral precordial leads (V4–V6) (a) and normal sinus rhythm after pharmacological cardioversion with no evidence of pre-excitation (b). ECG, electrocardiogram; SVT, supraventricular tachycardia.

Figure 2. Multimodality imaging performed on initial admission—echocardiography (a–d) and cardiac MRI ( e–i). Echocardiography demonstrated multiple echogenic homogeneous and hyperechoic masses, resembling rhabdomyoma (Rh), embedded into the left (LV) and right ventricular (RV) myocardium. Cardiac MRI demonstrates multiple intracardiac masses (arrows), with suspected aetiology rhabdomyoma related to TSC. TSC, tuberous sclerosis complex.
Table 1. Updated international tuberous sclerosis complex diagnostic criteria 2021 Reference Northrup, Aronow and Bebin4

TSC = tuberous sclerosis complex.
§ A pathogenic mutation is defined as a mutation that clearly inactivates the function of the TSC1 or TSC2 proteins, prevents protein synthesis, or is a missense mutation whose effect on protein function has been established by functional assessment.
¶ 1–3-mm hypopigmented macules scattered over regions of the body such as the arms and legs lines.
† Combination of the two major clinical features (LAM and angiomyolipomas) without other features does not meet the criteria for a definite diagnosis.
The patient was transferred to the neonatal ICU of a tertiary referral centre for further evaluation and management. The propranolol dosage was increased to 3 mg/kg/day and shortly thereafter lowered to 2 mg/kg/day secondary to hypotension. Multimodality imaging and genetic testing were performed in accordance with management recommendations for individuals with newly suspected tuberous sclerosis complex. Reference Northrup, Aronow and Bebin4 A cardiac MRI revealed multiple intracardiac masses that were mildly hyperintense on T2-weighted imaging (Fig. 2e–i). The largest mass was detected predominantly in the left ventricular cavity and extended from the basal to mid-anterior wall measuring 9.5 x 7.1 mm. A second mass measuring 6.5 x 3.6 mm was in the inferior left ventricular apical wall. The other three nodules were smaller (4.2 x 3.5 mm, 4.7 x 3.8 mm, and 4.4 x 2.7 mm) and located in the right ventricle (mid-anterior right ventricular septum, right ventricular apex, and right ventricular free wall, respectively). Following the administration of gadolinium-based contrast agents, these nodules showed mild enhancement, appearing less than the myocardium. Further radiological examination was performed to detect extracardiac lesions. Brain MRI was obtained to assess for the presence of tubers, subependymal nodules, migrational defects, or subependymal giant cell astrocytoma. The brain MRI demonstrated subependymal nodules and subcortical lesions. Additionally, abdominal ultrasonography revealed no significant abnormalities. Following the results from multimodality imaging, a definite diagnosis of tuberous sclerosis complex was made (definite tuberous sclerosis complex diagnosis [two major features: cardiac rhabdomyomas and subependymal nodules], Table 1). Results from the genetic investigation identified a pathogenic mutation in the tuberous sclerosis complex 1 gene, confirming the diagnosis of tuberous sclerosis based on the genetic diagnostic criteria (Table 1). At 5 days of age, supraventricular tachycardia reoccurred at a heart rate of about 220 bpm, which was resolved using non-invasive vagal manoeuvres. In response, propranolol administration frequency was changed to every 6 hours with the dosage remaining 2 mg/kg/day. Hereafter, the patient remained stable without further supraventricular tachycardia episodes, and the patient was ultimately discharged home. Due to the asymptomatic nature of the brain lesions, the patient received outpatient neurological follow-up. Cardiac clinical follow-up with electrocardiogram monitoring and interval echocardiographic assessments was carried out.
At 12 days of age, the patient was readmitted for supraventricular tachycardia recurrence with a heart rate of about 230 bpm at home, which was resolved with vagal manoeuvres performed by the parents. Following the failure of first-line pharmacological management, the patient was started on digoxin 8 mcg/kg/day (4 mcg/kg twice a day) in addition to the beta-blockers. Shortly after, the patient reported complications such as sleep disturbances, oral intolerance, and vomiting associated with digoxin treatment therefore digoxin was discontinued. Due to the recurring nature of supraventricular tachycardia, a loading dose of amiodarone 10 mg/kg/day (5 mg/kg twice a day) was given for 10 days. The patient remained haemodynamically stable and was discharged after a few days of observation in the hospital. At 3 weeks of age, the patient underwent clinical follow-up with electrocardiogram monitoring, and the amiodarone dosage was decreased to 5 mg/kg/day (5 mg/kg once a day). A week later, the patient had a recurrence of supraventricular tachycardia and after arrival in the emergency department continued to have multiple recurring supraventricular tachycardia episodes (Fig. 3a), which were resolved using non-invasive vagal manoeuvres (Fig. 3b). During the hospital admission, flecainide 3 mg/kg/day (1 mg/kg three times a day) was initiated in addition to propranolol and amiodarone. The patient remained stable on this regimen, with no more recurrence of supraventricular tachycardia, and was ultimately discharged home after 3 days. The patient underwent regular follow-up with electrocardiogram monitoring and had no more supraventricular tachycardia. After a 4-month period remaining stable on the therapeutic regimen, the patient was weaned off amiodarone and the propranolol dosage was increased to 3 mg/kg/day. Echocardiographic examination showed regression of cardiac rhabdomyomas. The patient remained asymptomatic from a cardiac standpoint at 1-year follow-up. Consequently, flecainide was discontinued. The rhabdomyomas regressed further on echocardiography. Finally, propranolol was discontinued, and the patient remained free from supraventricular tachycardia recurrence. In summary, the patient ceased all medications, discontinuing amiodarone at 5 months, flecainide at 1 year, and propranolol at 16 months. At present day (2 years and 9 months follow-up), there was continued regression of rhabdomyomas. The patient is followed once a year with future plans for an invasive electrophysiology procedure and potential ablation of the reentrant accessory pathway.

Figure 3. Patient ECG at second hospitalisation following the recurrence of SVT (a) and after cardioversion of SVT to sinus rhythm by vagal manoeuvres (b). ECG reveals a regular narrow-complex tachycardia with a heart rate of 205 bpm (A) and normal sinus rhythm after cardioversion using non-invasive vagal manoeuvres (B). ECG = electrocardiogram; SVT, supraventricular tachycardia.
Discussion
Arrhythmias are associated with the presence of cardiac tumours. A large retrospective review of patients with primary cardiac tumours revealed that of 106 patients with rhabdomyoma, 17 (16%) had clinically significant arrhythmia. Reference Miyake, Del Nido and Alexander6 Ten patients had pre-excitation, including two with recurrent sustained supraventricular tachycardia, while eight patients were asymptomatic. Reference Miyake, Del Nido and Alexander6 We present a case of recurrent supraventricular tachycardia without pre-excitation, of which few reports exist in the current literature. The exact mechanisms behind the occurrence of arrhythmic events are unclear but have been linked to the location of specific cardiac rhabdomyomas. Reference Hinton, Prakash, Romp, Krueger and Knilans7 Rather than typical pathways, abnormal atrioventricular connections have been demonstrated with the rhabdomyoma tumour tissue acting as a substrate. Reference Hinton, Prakash, Romp, Krueger and Knilans7 It has been proposed that rhabdomyoma cells are structurally identical to Purkinje’s cells and can thereby act as a potential accessory pathway. Reference Crome8 As a consequence of the regression of cardiac tumours, accessory pathways might dissipate, and arrhythmic events might cease. In our case, frequent echocardiographic follow-up showed continued regression. However, while the patient may be asymptomatic, interval assessment or ambulatory event monitoring is recommended in those with risk factors or the presence of abnormalities on electrocardiogram and echocardiography. Reference Hinton, Prakash, Romp, Krueger and Knilans7 Additionally, our patient exhibited symptoms of digoxin toxicity even with appropriate dosage perhaps due to decreased volume of distribution and plasma clearance of the drug related to clinically significant hypothyroidism.
A notable aspect of our case is the requirement of triple-drug therapy for management. A small series on combined therapy for refractory tachyarrhythmias consisting mostly of patients with structurally normal hearts demonstrated that in infants unsuccessfully treated with either flecainide or amiodarone in monotherapy, combination therapy resolved the tachyarrhythmias in 78% (seven out of nine patients) without occurrence of severe side effects. Reference Fenrich, Perry and Friedman9 Yet, the safety and efficacy of triple therapy have not been studied extensively. A single-centre retrospective observational study has described experience with triple therapy for refractory supraventricular tachycardias, using a combination of esmolol-propranolol, amiodarone, and flecainide in six patients with a success rate of 100%. Reference Ergül, Özyılmaz, Saygı, Tola, Akdeniz and Tuzcu10 These patients had abnormal haemodynamics and ventricular dysfunction requiring inotropic support, whereas the index patient in the present report had a functionally normal heart with the occurrence of supraventricular tachycardia due to structural abnormalities. There is a theoretical possibility of multiple concealed accessory pathways in the patient due to the multiple masses present, which only an electrophysiology study could have provided confirmation.
Conclusion
Appropriate management of arrythmias in the context of tuberous sclerosis complex requires multidisciplinary care. As the patient ages, arrhythmias tend to disappear, concomitant with regression of the tumours. Frequent cardiovascular surveillance for arrhythmia recurrence is suggested, even after the resolution of cardiac rhabdomyomas.
Acknowledgments
Xander Jacquemyn was supported by the Belgian American Educational Foundation.
Author contribution
XJ: Concept/design; data collection; data interpretation, drafting article, critical revision of article, and approval of article.
SK: Concept/design; data collection; data interpretation, drafting article, critical revision of article, and approval of article.
MIC: Concept/design; data collection; data interpretation, drafting article, critical revision of article, and approval of article.
KKM: Concept/design; data collection; data interpretation, drafting article, critical revision of article, and approval of article.
Financial support
None.
Competing interests
None.







