The Fontan procedure is the ultimate surgical stage of the univentricular pathway. Since its introduction in 1971, several modifications were made, from the atriopulmonary Fontan to cavopulmonary anastomosis, either by lateral tunnel or extracardiac conduit techniques. Despite being a surgical success, the Fontan procedure still remains a palliative treatment. The long-term impact of living with no subpulmonary ventricle is well known, becoming obvious that failure of the Fontan circulation is inevitable and the only definitive treatment relies on heart transplantation. Meanwhile, additional medical, surgical, or percutaneous management intend to delay, attenuate, or even rescue patients with “failing” Fontan physiology. Reference John1
The present study aimed to describe our experience with Fontan patients, in order to identify factors associated with the development of late failing Fontan circulation.
Demographic, anatomic, and perioperative characteristics on the medical records of Fontan patients, born after 1987, and followed up in our centre were reviewed. Patients with early-postoperative failing were excluded. Late failing was defined as: Fontan conversion, heart failure symptoms, protein-losing enteropathy, plastic bronchitis, death, or cardiac transplant.
Fifty-two patients were included, 28 males (54%), with a median (25th–75th percentile, P25–P75) age of 18.9 (15.4–20.2) years at the last follow-up visit. Thirty-three patients had single left ventricle anatomy, 15 single right ventricle anatomy, and 4 biventricular heart. The Fontan procedure was performed at a median (P25–P75) age of 4.6 (4.0–8.1) years. Forty-seven patients had an extracardiac conduit, and 5 a lateral tunnel, while 44 were fenestrated. No atriopulmonary Fontan was performed (see Table 1).
Table 1. Baseline characteristics of all patients, non-“Failing” Fontan and “Failing” Fontan patients.
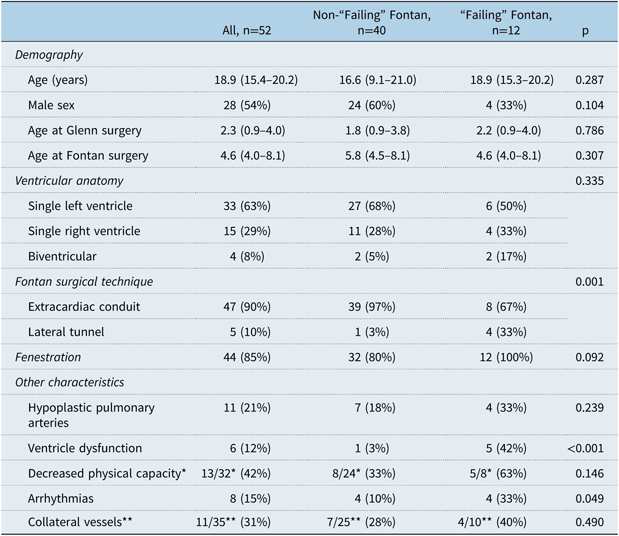
* Not all patients were able to performed a treadmill exercise test
** Not all patients had confirmation of collateral vessels by cardiac catheterization, CT or MRI
Twelve patients developed late failing (five protein-losing enteropathy, five heart failure symptoms, one plastic bronchitis, and one death – malignant arrhythmia, 5.2 years after Fontan surgery) at a median duration (P25–P75) of 7.2 (1.4–10.4) years after Fontan. No patients underwent cardiac transplant or Fontan conversion. Compared with non-failing patients, the failing Fontan group presented more frequent post-operative arrhythmias [4/12 (33%) versus 4/40 (10%), p=0.049], ventricular dysfunction [5/12 (42%) versus 1/40 (3%), p<0.001], and lateral tunnel [4/12 (33%) versus 1/40 (3%), p=0.001], although the extracardiac conduit was performed in more than half (8/12, 67%) of the failing patients. Additionally, lateral tunnel significantly increased the risk of failing Fontan [odds ratio 19.5 (95% CI, 1.9–198.3), p=0.012], as well as having arrhythmias [odds ratio 27.9 (95% CI, 2.8–275.9), p=0.004]. No differences were found regarding the age at Glenn or Fontan surgery, ventricular anatomy, pulmonary artery size, decreased physical capacity, and presence of fenestration or collateral vessels.
In conclusion, our patients presented a favourable evolution with <25% progressing to late failing Fontan. The lack of association between some of the studied variables might be justified by the small sample. Future studies on the factors predicting the outcome of univentricular pathway are needed. Most of the patients adapt to this long-standing haemodynamic state and only become symptomatic at a later stage. Therefore, it is crucial to identify “high-risk” Fontan survivors that will benefit from preventive and early interventions, in order to optimise their clinical management and prevent serious complications.
Acknowledgements
Authors may also wish to acknowledge individuals, who are not named as authors, who have contributed materials, expertise, or time to the study.
Financial Support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of Interest
None.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008, and has been approved by the institutional committees.



