Historical standard surgical correction of tetralogy of Fallot has always involved a transannular incision with subsequent patch enlargement (transannular patch) in order to close the ventricular septal defect and adequately relieve right ventricular outflow tract obstruction. Reference Gott1 However, as these patients had been followed for a period of time, deleterious effects on cardiac function and volume were found as consequences of large ventriculotomy incision and late pulmonary regurgitation.
As a result, an alternative surgical approach was conceived which omitted the transannular incision and opted for a transatrial-transpulmonary approach Reference Pacifico, Sand, Bargeron and Colvin2–Reference Boni, García and Galletti7 to close the ventricular septal defect and perform infundibulectomy. The pulmonary valve annulus could be preserved (pulmonary valve sparing; PVS), and the results were convincing with significantly lower incidence of late pulmonary regurgitation and right ventricular dilation and dysfunction. However, some limitations existed which included the potential residual right ventricular outflow tract and pulmonary valve stenosis after repair and the age limitation in which this approach would be more suitable for larger infants. Therefore, a right ventriculotomy was sometimes mandated albeit with potentially negative effects on right ventricular function. There had been few studies conducted on this matter and, to our knowledge, none that included patients diagnosed with double outlet right ventricle with pulmonary stenosis whose surgical repair approach was nearly identical. Therefore, we primarily aimed to compare the impact of right ventriculotomy on cardiac function as well as patients’ symptoms on follow-up after pulmonary valve-sparing repair of tetralogy of Fallot and double outlet right ventricle with pulmonary stenosis. Our secondary outcomes focused on the potentially negative effects of systemic-to-pulmonary shunt on left ventricular function as some of the patients aimed for pulmonary valve-sparing repair might be more in need of a staged operation as they had to grow larger to be considered eligible for this type of repair. Also, we aimed to compare echocardiographic parameters, specifically peak pressure gradient across pulmonary valve, between before-discharge and at latest follow-up. We hypothesised that the intact pulmonary valve annulus, which was the key point of pulmonary valve-sparing repair, should have the tendency to grow further. This should theoretically be demonstrated by a reduction in peak pressure gradient across pulmonary valve when followed on a long-term basis.
Materials and methods
Data collection
The study protocol and ethical issues were reviewed and approved by Human Research Ethics Committee, Faculty of Medicine, Ramathibodi Hospital, Mahidol University, Bangkok, Thailand. We retrospectively collected data from electronic medical records of Ramathibodi Hospital from 1st January 2013 to 31st October 2023. The initial number of patients diagnosed with tetralogy of Fallot and double outlet right ventricle with pulmonary stenosis who underwent total corrections was 116. Patients with other significant co-existing cardiac anomalies, who underwent other types of repairs (including transannular patch and conduit reconstruction), and whose medical record data were significantly missing (such as no operative notes, no follow-up visits) were excluded from our study. Ultimately, 67 patients were excluded (5 from major co-existing cardiac anomalies, 47 from undergoing other types of repairs, and 15 from significantly insufficient recorded data), leading to a total of 49 patients enrolled.
Preoperative (including general demographic data as well as pulmonary valve z-score and morphology), operative (including operative time, cardiopulmonary bypass time, aortic cross-clamp time, operative techniques, and post-repair pressure measurements), and postoperative details (including ICU stay duration, hospital stay duration, symptoms, and follow-up echocardiographic parameters) were collected. Specific echocardiographic parameters acquired included left ventricular ejection fraction, left ventricular end-systolic volume index, left ventricular end-diastolic volume index, tricuspid annular plane systolic excursion, and peak systolic pressure gradient across pulmonary valve. In our institution, we calculated body surface area using Mosteller formula.
Indications of surgery and surgical techniques
The indication of pulmonary valve-sparing repair in our institution was preoperative echocardiographic pulmonary valve z-score of more than −4 with acceptable valve morphology (amendable to adequate repairs and orifice size-increasing manoeuvres such as commissurotomy or dilation). After repair completion and weaning off bypass, a right ventricular to left ventricular pressure ratio of more than 0.7 with unstable haemodynamics and no residual lesions (e.g. ventricular septal defects) would mean reinstitution of bypass with transannular patch subsequently placed. In our institution, we would consider performing subsequent ventriculotomy with patch enlargement after pulmonary valve-sparing repair when adequate right ventricular outflow tract muscle resection could not be achieved via transatrial route alone, right ventricular outflow tract z-score was less than −4 as measured intraoperatively, and possible right ventricular outflow tract obstruction from intracardiac baffle for ventricular septal defect closure was anticipated, especially in double outlet right ventricle with pulmonary stenosis.
Principles of reparative techniques were generally the same in all patients. Standard median sternotomy with partial thymectomy was performed, and cardiopulmonary bypass was established using aorto-bicaval (or tricaval in case of persistent left superior caval vein) cannulation with moderate hypothermia (28°C). Custodiol® cardioplegic solution was used in all cases with repeated doses as appropriate. Left ventricular venting was done via interatrial septum after aortic cross-clamping. Right atriotomy was performed first after cardioplegic arrest. The main pulmonary artery incision would then be made longitudinally starting from near the pulmonary bifurcation down to just above the pulmonary valve annulus. Pulmonary valve would be examined through the main pulmonary arteriotomy and subsequently dilated or repaired as appropriate. We used Hegar dilators for annular dilation and performed commissurotomy or valvuloplasty with autologous pericardial patch for repairs. The obstructing septomarginal and septoparietal trabeculations would be divided via the main pulmonary arteriotomy as well as the right atriotomy. If initial muscle resection was thought to be inadequate, a limited (short) right infundibulotomy would be made just below the pulmonary valve annulus and further muscle resection performed through this incision. After completion of muscle resection, the ventricular septal defect would be closed via the right atriotomy with either a Dacron patch or a GORE-TEX® graft. The main pulmonary arteriotomy and, if any, the right ventriculotomy wound be closed using autologous or bovine pericardial patch, completing the repair (Fig 1). Patients would be rewarmed and weaned off bypass in a standard manner. Modified ultrafiltration was used in all cases.

Figure 1. (a) External anatomy of TOF before repair (in this case, with an existing right modified Blalock-Taussig-Thomas shunt [RMBTTS] in place) (b) Main pulmonary artery and ventriculotomy patches in place after completion of pulmonary valve sparing repair with ventriculotomy.
Follow-up
Postoperative echocardiographic evaluation was performed before discharge and at a 6-month to 1-year interval. Subsequent evaluation would be performed half-yearly or yearly thereafter. General symptoms and NYHA class assessment were evaluated during out-patient department visits by the attending physicians at every follow-up period.
Statistical analysis
Patient characteristics, operative, and postoperative details with continuous variables were compared using Wilcoxon rank-sum (Mann–Whitney) test while categorical variables were compared with chi-square test. Echocardiographic parameters before discharge and at latest follow-up were compared using Wilcoxon signed-rank test. P-value of less than 0.05 was considered statistically significant. The statistical software used was Stata version 14.1.
Results
Preoperative details (Table 1)
There were 49 patients enrolled in our study, with 10 in pulmonary valve sparing with ventriculotomy and 39 in without ventriculotomy group. Overall patient characteristics were generally similar between both groups. Sub-diagnoses were less prevalent in pulmonary valve sparing without ventriculotomy group. Also, preoperative haemoglobin level was also significantly higher in pulmonary valve sparing with ventriculotomy group (15.65 versus 13 g/dl, P < 0.05). Pulmonary valve z-score was significantly lower and pre-repair annular diameter was significantly smaller in pulmonary valve sparing with ventriculotomy group compared to without ventriculotomy group (−1.18 versus −0.04 and 11.25 versus 13 mm, P < 0.05, respectively).
Table 1. Preoperative details

Legends: PVS = pulmonary valve sparing; TOF = tetralogy of Fallot; DORV/PS = double outlet right ventricle with pulmonary stenosis; PDA = patent ductus arteriosus; ASD = atrial septal defect; LSVC = left superior caval vein; IQR = interquartile range; RVOT = right ventricular outflow tract.
Operative details (Table 2)
Patients who underwent pulmonary valve-sparing repair with ventriculotomy had significantly longer aortic cross-clamp and cardiopulmonary bypass time compared to without ventriculotomy (92 versus 75 minutes and 123 versus 102 minutes, P < 0.05, respectively). Also, they required more pulmonary valve repair manoeuvres. Post-repair annular diameter and right ventricular pressure/left ventricular pressure ratio were statistically similar between both groups (0.57 versus 0.5, P = 0.08).
Table 2. Intraoperative details
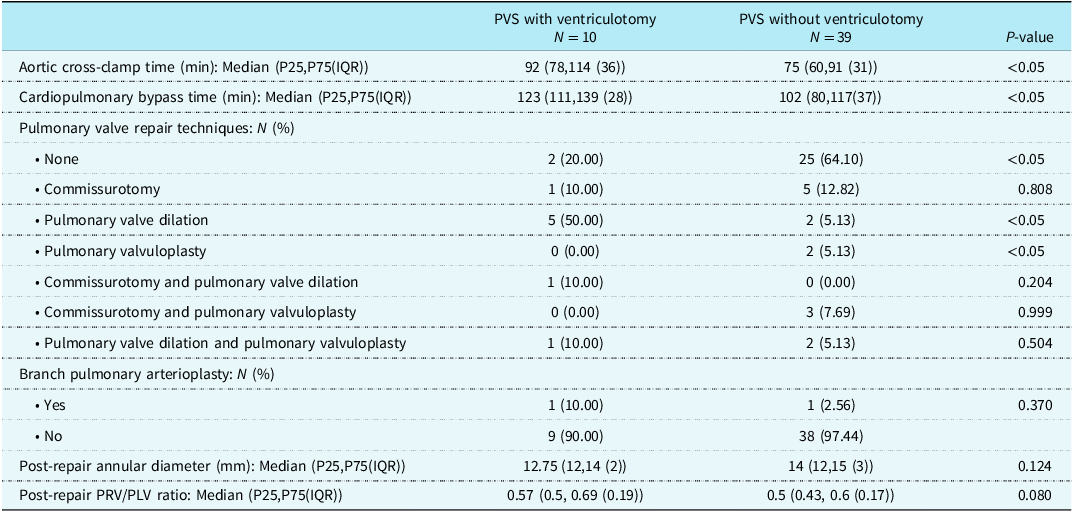
Legends: PVS = pulmonary valve sparing; IQR = interquartile range; PRV/PLV = right ventricular pressure/left ventricular pressure.
Postoperative details and follow-up (Table 3)
General postoperative care measures were similar between both groups, except for mechanical ventilation time which was longer in pulmonary valve sparing with ventriculotomy group (19.5 versus 12 hours, P < 0.05). Latest follow-up and echocardiography period were also statistically similar (42 versus 71 months, P = 0.108 and 37 versus 54 months, P = 0.524, respectively). Except for before-discharge left ventricular ejection fraction, all echocardiographic parameters both before-discharge and at latest follow-up were also generally similar between both groups.
Table 3. Postoperative details and follow-up

Legends: PVS = pulmonary valve sparing; IQR = interquartile range; LVEF = left ventricular ejection fraction; LVESVi = left ventricular end-systolic volume index; LVEDVi = left ventricular end-diastolic volume index; TAPSE = tricuspid annular plane systolic excursion; PSPG = peak systolic pressure gradient.
Effects of systemic-to-pulmonary artery shunt (staged operation) on left ventricular function (Table 4)
All echocardiographic parameters on left ventricular function were statistically similar between patients who underwent staged operation (required previous systemic-to-pulmonary shunt) and total repair as a primary operation.
Table 4. Effects of systemic-to-pulmonary artery shunt (staged operation) on left ventricular function.

Legends: LVEF = left ventricular ejection fraction; LVESVi = left ventricular end-systolic volume index; LVEDVi = left ventricular end-diastolic volume index.
Echocardiographic parameters before discharge and at latest follow-up (Table 5)
Right and left ventricular function generally improved and peak systolic pressure gradient across pulmonary valve also significantly reduced (20 versus 11 mmHg, P < 0.05) compared between before-discharge and at latest follow-up.
Table 5. Echocardiographic parameters before discharge and at latest follow-up.

Legends: LVEF = left ventricular ejection fraction; LVESVi = left ventricular end-systolic volume index; LVEDVi = left ventricular end-diastolic volume index; TAPSE = tricuspid annular plane systolic excursion; PSPG = peak systolic pressure gradient.
Discussion
Rationale behind avoidance of transannular patch
Since its first explanation in the 1880s, surgical treatment of tetralogy of Fallot could only be in the form of a palliative systemic-to-pulmonary artery shunt creation as the needed corrective surgical procedures inside a human heart were still impossible. In March 1954, Lillehei and colleagues performed the first total correction of tetralogy of Fallot using cross-circulation with remarkable results. Reference Gott1 Their techniques involved a large, transannular right ventriculotomy incision to close the ventricular septal defect and perform infundibulectomy to relieve right ventricular outflow tract obstruction with the ventriculotomy subsequently closed primarily. Their short-term results were satisfying, and the technique has become the standard practice for tetralogy of Fallot repair. In order to further enlarge the right ventricular outflow tract, a transannular patch could be applied, closing the incision from the main pulmonary artery, anterior pulmonary annulus, and down to the right ventriculotomy incision. However, when followed on a long-term basis, these patients exhibited signs and symptoms of right ventricular failure as well as life-threatening arrhythmias. As the name suggested, transannular patch, which included incision through the pulmonary annulus, would inevitably result in late pulmonary regurgitation. This, coupled with a large right ventriculotomy incision, resulted in progressive right ventricular dilation and dysfunction. An abnormal interventricular interaction would occur and left ventricular function subsequently deteriorated. The large patch was also non-contractile and could become aneurysmal, resulting in ineffective forward pumping of the right ventricle (“energy sink”). Scar surrounding the ventriculotomy could also become a nidus for malignant ventricular arrhythmias. These patients would eventually be in need of pulmonary valve replacement in order to halt or reverse these delirious processes.
Advantages of pulmonary valve-sparing repair
Armed with the aforementioned knowledge, a new approach of pulmonary valve-sparing repair was proposed. A transatrial-transpulmonary approach, Reference Pacifico, Sand, Bargeron and Colvin2,Reference Padalino, Vida and Stellin3 by its name, utilised a right atriotomy and a pulmonary arteriotomy to approach and close the ventricular septal defect and perform infundibulectomy. The technique refrained from pulmonary annular incision and thus better-preserved long-term pulmonary valve function, resulting in less incidence of late pulmonary regurgitation and superior global cardiac function on a long-term basis. This technique has become more widespread with better long-term results and has also been adopted in our institution for more than a decade. We advocated the use of pulmonary valve-sparing repairs as we strongly believed that every effort should be made to preserve the pulmonary valve integrity whenever possible in order to minimise late pulmonary valve regurgitation and its deleterious effects. In our institution, we utilised preoperative echocardiographic assessment of pulmonary valve z-score Reference Stewart, Backer, Young and Mavroudis4,Reference Lopez, Colan and Stylianou5 as a primary indicator to proceed with this repair technique. A z-score of more than -4 would be considered suitable for pulmonary valve-sparing repair. Pulmonary valve morphology was also assessed and, if abnormal, subsequently repaired whenever possible. Reference Bacha6 At repair completion, right ventricular pressure/left ventricular pressure ratio would be calculated and a ratio of less than 0.7 was considered acceptable in our institution. Higher ratios warranted a thorough search for residual obstructive lesions which must be aggressively addressed. Some groups Reference Stewart, Backer, Young and Mavroudis4,Reference Boni, García and Galletti7,Reference Siddiqi, Adewale and Pena8 considered a ratio threshold of 0.75 or utilised a pressure gradient across pulmonary valve of not more than 45 mmHg to be adequate. We considered these numbers to be very high and their patients often had more difficulty in weaning-off bypass and more turbulent postoperative courses, which were similar to our past experience. Our results showed that the average preoperative pulmonary valve z-scores were −1.18 and −0.04 and post-repair right ventricular pressure/left ventricular pressure ratios were 0.57 and 0.5 for pulmonary valve-sparing repair with and without ventriculotomy, respectively. These were considered very acceptable for pulmonary valve-sparing repair, resulting in our good overall long-term outcomes. Despite having a theoretically more difficult early postoperative course than transannular patch repair due to potential residual outflow obstruction and also post-bypass effects such as pulmonary hypertension which could result in more postoperative right ventricular dysfunction, Reference Bacha6 one study Reference Touré, Roubertie and Bridier9 suggested otherwise. Their results showed that repairs using pulmonary valve-sparing technique reduced the length of stay in ICU with even less inotropic use as compared to transannular patch repairs. We agreed with their results as we believed that optimal postoperative care measures, although potentially more complicated, could result in comparable immediate outcomes. More importantly, pulmonary valve-sparing technique with its lower incidence of pulmonary valve regurgitation and subsequent right ventricular dysfunction would ultimately result in better long-term outcomes, as proved in our series.
Pulmonary valve-sparing procedures and limitations
Certain limitations existed in pulmonary valve-sparing repair techniques. Pulmonary valve annulus size and morphology were among them and efforts were made at improving repair techniques without having to resort to transannular incision. Reference Lozano-Balseiro, Garcia-Vieites and Martínez-Bendayán10–Reference Hoashi, Kagisaki and Meng12 Dysplastic valves were considered as precaution but not preclusion to pulmonary valve-sparing repair. In our institution, we sometimes performed valvuloplasty using autologous pericardium to extend the cusp length if considered inadequate. Also, commissurotomy and sometimes dilation with Hegar dilators were performed. We did not utilise balloon dilation as a means of repairs due to equipment limitations. Our cohort included a considerable number of patients undergoing pulmonary valve repair with remarkable long-term outcomes. However, we advised against extensive pulmonary valve repair to enlarge the annulus as this could potentially lead to pulmonary regurgitation on a long-term basis. Our results contained a considerable number of patients undergoing various types of repairs but with minimal changes in post-repair annulus size. Despite this, our outcomes were still justified and therefore only limited repairs as necessary were encouraged.
Because of pulmonary valve annulus size limitation, pulmonary valve-sparing repair would generally be considered justified in larger infants rather than young infants or neonates. The elapsed time from diagnosis to correction meant that some patients would be in need of a systemic-to-pulmonary artery shunt in order to relieve their cyanosis. This shunt could result in increased left ventricular volume load and possible dysfunction. Our cohorts contained a considerable number of patients who had a systemic-to-pulmonary artery shunt created before total repair. When compared to patients without previous systemic-to-pulmonary artery shunt, we did not find cardiac function on follow-up to be different. This suggested that if systemic-to-pulmonary artery shunt was needed, it could be safely performed Reference Kobayashi, Kotani, Kuroko, Kawabata, Sano and Kasahara13 without jeopardising future left ventricular function.
Right ventriculotomy in conjunction with pulmonary valve-sparing repair
Even with its superior results Reference van den Berg, Hop and Strengers14 and several pulmonary valve repair techniques available, pulmonary valve-sparing repair still pertained an Achilles’ heel. A transatrial-transpulmonary approach did not include a right ventriculotomy incision. In case of severe infundibular stenosis or small, narrow infundibulum, only muscle resection from the inside might be of inadequate relief of obstruction. Therefore, an additional small right ventriculotomy (albeit without transannular incision) with patch augmentation might be required. This manoeuvre, although without breaching pulmonary valve annulus integrity, came with a cost of potential long-term right ventricular dysfunction. Only a few studies focused on this aspect and one recently published review Reference Ono, Hoashi and Imai15 suggested that right ventriculotomy for pulmonary valve-sparing repair of tetralogy of Fallot did not cause major events in terms of mortality, reoperation or reintervention, as well as arrhythmias. This study also included data on postoperative echocardiography and their results supported ours in which there were no differences in cardiac function and volume as well as ICU and total hospital stay duration compared between pulmonary valve sparing with and without ventriculotomy. They also emphasised on maximal limitation of right ventriculotomy incision length whenever possible. We believed that the more important contributing factor of right ventricular dysfunction was the severity of pulmonary valve regurgitation, not the small ventriculotomy incision. Moreover, this incision would be normally made at the infundibulum, not the right ventricular body, which did not contribute greatly to contractile function. From this knowledge, it could be strongly suggested that pulmonary valve annulus should be preserved whenever deemed possible. In our institution, we also routinely limited right ventriculotomy incision length to no more than one-third the distance from the pulmonary annulus to the base of the right ventricle, or 2 cm at most. This might not greatly benefit future right ventricular function according to the results, but still further studies on this aspect should be made.
Somatic growth of pulmonary valve annulus following pulmonary valve-sparing repair
Another advantage of leaving the pulmonary annulus intact was its ability to further grow. Reference Ito, Ota and Murata16,Reference Leobon, Cousin and Hadeed17 Some studies Reference Lee, Lee and Kwak18–Reference Talwar, Anand, Siddarth, Ramakrishnan, Choudhary and Airan20 obtained acceptable outcomes of pulmonary valve-sparing repair in terms of pulmonary valve and subsequent right ventricular function. They suggested that even with immediate postoperative residual right ventricular outflow tract gradient with pulmonary valve-sparing repair, the pulmonary valve still had tendency to grow and this was observed as a reduction of gradient on follow-up. Our results demonstrated a significant reduction in peak systolic pressure gradient across pulmonary valve compared between pre-discharge and the most recent echocardiography. Techniques of optimal pulmonary valve-sparing repair with good long-term results have been discussed Reference Morales, Zafar and Fraser21,Reference Morales, Zafar and Heinle22 with cardiac MRI Reference Davlouros, Kilner and Hornung23,Reference Puranik, Tsang and Lurz24 also emerging as a new standard tool for evaluation of postoperative cardiac function. We did not perform cardiac MRI in every patient postoperatively and therefore, the results were not included in our study. In our institution, we consistently aimed to increase utilisation of cardiac MRI in our patients and hoped that one day, this would eventually become a routine practice.
Limitations
The limited number of patients included alongside being a retrospective trial were the major limitations of our study. Also, we did not have a regularly scheduled echocardiography follow-up appointment protocol in our institution. This was mainly due to the availability of the echocardiography laboratory and also the attending paediatric cardiologists. Another limitation regarding echocardiography was the recorded parameters in which some were either not mentioned or lost, resulting in incomplete postoperative echocardiographic parameters for our final analysis. Lastly, we did not use cardiac MRI, which was considered to be the standard method, to assess postoperative cardiac function.
Conclusions
Right ventriculotomy in pulmonary valve-sparing repair of tetralogy of Fallot or double outlet right ventricle with pulmonary stenosis did not result in worse cardiac function and symptoms on follow-up when compared to without ventriculotomy. This finding suggested that the more important contributing factor to ventricular function was pulmonary valve function and its integrity should be preserved whenever possible. Right ventriculotomy would be justified as an acceptable means to further relieve right ventricular outflow tract obstruction if necessary. Therefore, if surgeons were confident in the adequacy of infundibulectomy yet with significant remaining post-repair subvalvular gradient, a ventriculotomy patch could then be applied without hesitation.
Availability of data and materials
None.
Acknowledgements
I would like to express my gratitude to my corresponding author and advisor, Piya Samankatiwat, M.D., M.Sc., for his expertise and assistance throughout all aspects of the study.
Authors’ contribution
Khunthorn Kadeetham, M.D. : concept and design, data correction, review the data, analysis and interpretation of data, drafting and revising the article, final approval.
Piya Samankatiwat, M.D., M.Sc. : data correction, review the data, revising the article, final approval.
Financial support
The research received no specific grants from any funding agency in the public, commercial, or non-for-profit sectors.
Competing interests
Authors have nothing to disclose with regard to commercial support.
Ethical standards
The study protocol and ethical issues were reviewed and approved by Human Research Ethics Committee, Faculty of Medicine, Ramathibodi Hospital, Mahidol University, Bangkok, Thailand (No. MURA2023/939).









