Introduction
Since its first report as a surgical “repair” for tricuspid atresia with an underdeveloped dysfunctional right ventricle, Reference Fontan and Baudet1 the Fontan operation has evolved and improved through decades, Reference Azzolina, Eufrate and Pensa2–Reference Padalino, Ponzoni and Castaldi6 now with a very low perioperative mortality and good long-term survival. Reference Schilling, Dalziel and Nunn7–Reference Pundi, Johnson and Dearani9 Surgical indications have been gradually extended to include all complex CHDs in which only one ventricle is fully developed (functional single ventricle). Thus, the heterogeneous population of Fontan patients currently includes those who have more than one ventricle, or even two ventricles of adequate size, but not amenable to biventricular repair. Reference Honjo10 As a consequence, the role of ventricular morphology Reference Ponzoni, Azzolina and Vedovelli11–Reference Julsrud, Weigel and Son13 and of the additional ventricular chamber Reference Rossi, Frigo and Reffo14 on clinical outcomes after the Fontan operation have been studied. Specifically, two questions that remain are: (1) Is a functional single morphologically left ventricle better than a functional single morphologically right ventricle? (2) Is a ‘two-ventricle’ Fontan better than a ‘single-ventricle’ Fontan?
We herein report a single-centre analysis of a cohort of Fontan patients who underwent a cardiac MRI study to assess whether dominant ventricular morphology and the presence and size of an additional ventricular chamber may correlate to clinical and functional outcomes.
Materials and methods
Study population
This is a single-centre retrospective observational study including patients with functional single ventricle who underwent a Fontan procedure between January 1980 and March 2019. A review of medical records was approved by the hospital committee for clinical investigation (protocol 59005). Individual patients were de-identified, and the need for patient consent was waived.
Patients who underwent both a cardiac MRI and a cardiopulmonary exercise test between 1 January, 2020 and 31 December, 2022 were included. Patients with no data about additional ventricular chamber or with significant atrioventricular valve dysfunction (i.e., regurgitation equal to or greater than moderate, to avoid over-estimation of ventricular ejection fraction) were excluded. Data were collected via electronic medical records review and telephone interviews.
CMRI analysis
We retrospectively analysed cardiac MRI to confirm dominant ventricle morphology and assess the volumes of both the dominant ventricular chamber, and the additional ventricular chamber, if present. All cardiac MRI studies were performed using an Achieva 1.5 T scanner (Philips Healthcare; Best, the Netherlands). Acquisition protocols included cine imaging of the functional single ventricle short axis, consisting of a stack of 12–17 contiguous slices and late gadolinium-enhancement imaging in short-axis and four-chamber planes.
Ventricular morphology (right vs. left) was defined according to the presence/absence of smooth endocardial surface of the interventricular septum, chordae inserting into the interventricular septum, a triangular versus ellipsoid-shaped ventricular cavity, the presence/absence of a moderator band, and a bicuspid versus tricuspid appearance of the atrioventricular valve. Reference Foale, Stefanini, Rickards and Somerville15 The dominant ventricular chamber was the one having the larger size and receiving most of the ventricular inflow, while the smaller one was designated as the additional ventricular chamber. In patients whose additional ventricular chamber had adequate dimensions for biventricular repair, the classification of the primary ventricle and the additional ventricular chamber was adjudicated according to the presence of a patent connection to the aorta directly or through a ventricular septal defect. We classified the additional ventricular chamber in two groups according to a volume greater or lower than 20 mL/m2. We set this single cut-off to distinguish rudimentary additional ventricular chambers versus additional ventricular chambers closest to normal volumetry for the patient’s body size, since patients whose additional ventricular chamber is ≥25 mL/m2 are usually considered eligible for biventricular repair (at the time of surgical repair/palliation) 0.10 Since less consensus is available to support measuring the additional ventricular chamber as a percentage of the dominant ventricle, we preferred to express the volume as mL/m2. The presence of fibrosis (late gadolinium enhancement) in the dominant ventricle was localised using a 17-segment ventricular model, 16 and its extent was described as absent, or involving <20%, 20–40%, or >40% of functional single ventricle segments. Additional methods are included in Supplemental Methods.
Cardiopulmonary exercise testing
A symptom-limited cardiopulmonary exercise test was performed on a cycle-ergometer (Cycle-ergometer eBike, General Electrics) or a treadmill (COSMOS model T170 DE-med), according to a modified Bruce protocol. The ramp test protocol was adapted based on the characteristics of each patient and their reported level of physical activity, with the aim of reaching exhaustion within 8–12 minutes of incremental exercise. Respiratory gas exchange variables were measured continuously throughout resting and the exercise period, using a MasterScreen CPX diagnostic system (CareFusion, Hoechberg, Germany). The peak oxygen consumption (pVO2) was defined as the highest value of VO2 attained in a 30-second interval at peak exercise. Workload was measured as metabolic equivalents.
Statistical analysis
Descriptive statistics were reported as median and interquartile range for continuous variables and as percentages (absolute numbers) for categorical variables. Wilcoxon and chi-squared tests were carried out to compare continuous and categorical variables, respectively. Poisson incidence rates (per 1000 person-years) of late adverse events were computed. The p-values and alpha level have been adjusted for false discovery rate inflation via Benjamini Hochberg correction.
The Propensity Score estimation analysis was performed to assess the impact of the dominant ventricular morphology and the size of the additional ventricular chamber on the post-operative endpoints. The clinical characteristics of the patients in the different exposure groups were balanced by performing an inverse of treatment weight estimation procedure. Reference Chesnaye, Stel and Tripepi17 The Propensity Score was calculated by accounting for potential confounding factors, including age at surgery, heterotaxy syndrome, presence of a fenestration, total cavo-pulmonary connection type, need for neonatal palliation, need for neonatal cardiopulmonary bypass, or aortic cross-clamping. The Covariate Balancing Propensity Score estimation method was considered for the computation. Reference Fong, Ratkovic and Imai18 The balancing of the covariate was visually assessed by reporting the standardised mean differences across groups before and after the Propensity Score adjustment. Standardised mean difference lying in absolute values in 0.1 thresholds indicates a suitable post-Propensity Score adjustment confounding balancing. Reference Austin19 The inverse of treatment weight Gamma model was considered to account for the skewness in the outcome distribution; the Shapiro–Wilk test was computed to assess the normality of the outcomes. The linear regression was instead computed for the normally distributed endpoint. The computations were performed with the R 3.4.2 System 20 using WeightIt Reference Greifer21 and Rms packages. Reference Harrell22
Results
As shown in Figure 1, among 280 Fontan patients operated on between 1980 and 2019, we included 50 patients eligible for this study (median age at enrolment [years]: 20, interquartile range 16–26; range 9–47; median length of follow-up [years]: 16, interquartile range 11.5–23; range 4–42). Complete pre-operative and post-operative patient characteristics are shown in detail in Supplementary Tables S1–S3. Twenty-nine patients (58%) had functional single left ventricle, and 21 had functional single right ventricle (42%, Table S1). Tricuspid atresia (26%) and hypoplastic left heart syndrome (22%) were the most frequent diagnoses. The median age and weight at Fontan completion were 36 months (interquartile range 28–48) and 13 kg (interquartile range 12–15), respectively. Seventy-eight percent of patients required neonatal palliation (among these, Norwood operation in 6, Damus-Kaye-Stansel in 3, and other arch reconstructions in 4). Cardiopulmonary bypass and cross clamping through the Fontan staging were used in 1/3, and three patients underwent a Damus-Kaye-Stansel at the second stage. The most common Fontan completion was the extracardiac conduit, and fenestration was performed in 74% (Table S1). Median ICU and hospital stay were 3 days (interquartile range 2–5) and 17 days (interquartile range 12–23), respectively. The most frequent early adverse event was low cardiac output syndrome in 3 patients (6%), two of whom required extracorporeal membrane oxygenation support (Table S2).

Figure 1. Flowchart of patient selection.
At a median follow-up of 16 years (interquartile range 11.5–23, Table S3), 47 patients (94%) presented with NYHA classes I-II. Twenty-four patients were on anti-congestive medical therapy (ACE inhibitors at usual dosage according to BSA), and 6 were on pulmonary vasodilator(s) (Sildenafil and/or Bosentan, as a long-term therapy, after confirmation of pulmonary arterial hypertension on cardiac catheterisation, defined as transpulmonary gradient >6 mmHg or pulmonary vascular resistance index >3 WUm Reference Azzolina, Eufrate and Pensa2 as suggested by the European Pediatric Pulmonary Vascular Disease Network Reference Hansmann, Koestenberger and Alastalo23 ). Two patients (4%) required transcatheter interventions for significant stenosis in the pulmonary arteries. Three patients (7%) presented with NYHA classes III or IV, two of whom died for failing Fontan (14 and 44 years, respectively). Four (9%) underwent a Fontan conversion surgery to extracardiac conduit, one (2%) is on the transplantation waiting list, and another (2%) received a transplant.
On cardiac MRI evaluation, the mean indexed end-diastolic volume (EDV) of the dominant ventricle was 90 ± 25 mL/m2. An additional ventricular chamber was present in all patients, and it was <20 mL/m2 in 38/50 (76%) cases and ≥20 mL/m2 in 12/50 (24%, Table S4). An additional ventricular chamber larger than 25 mL/m2 was present only in 3/50 patients (6%), and it was always smaller than 35 mL/m2, since our additional ventricular chamber cut-off for biventricular repair eligibility (in absence of other contraindications) is >25 mL/m2. Thirty-nine patients had available late gadolinium enhancement imaging data. Among these, 12/39 (31%) had no myocardial fibrosis in the dominant ventricle, while it was minimal (<20%) in the others. Only one patient had extended fibrosis (>40% of the segments).
Cardiopulmonary exercise test showed an overall mean pVO2 of 1570 mL/min (± 456), and a mean pVO2/kg of 29.3 mL/kg/min (± 6.7). The mean peak metabolic equivalents was 13.3 (± 2.5) (Table S4).
Association between functional single ventricle morphology and clinical outcomes
As described in Table 1, early post-operative low cardiac output syndrome occurred more frequently in patients with functional single right ventricle (p = 0.043), who, in the long term, were also more likely to require therapy for heart failure (p = 0.05). There were no other significant differences in early or late outcomes between patients with functional single left ventricle and functional single right ventricle.
Table 1. Impact of ventricular morphology of the dominant ventricle on clinical outcomes and cardiac MRI and cardiopulmonary exercise test metrics

CPB = cardiopulmonary bypass; CC = cross-clamping; AVC = additional ventricular chamber; CMRI = cardiac MRI; CPET = cardiopulmonary exercise test; EDVI = indexed end-diastolic volume; FSLV = functional single left ventricle; FSRV = functional single right ventricle; IQR = interquartile range; LGE = late gadolinium enhancement; MET = metabolic equivalents. *Information regarding home therapy was not available in five patients.
Interestingly, functional single left ventricle was associated with a significantly better cardiac function at follow-up (median ejection fraction 56% [interquartile range 51–62] vs. 51% [v 44–56], p = 0.04), and lower prevalence and extent of fibrosis of the dominant ventricle (absent LGE in 50% vs. 6%, p < 0.025). Furthermore, patients with functional single left ventricle displayed a better functional capacity at CPET with a median peak metabolic equivalents equal to 14.1 (interquartile range 12.9–16), versus 12.3 in functional single right ventricle (interquartile range 10.9–12.6, p = 0.01), and a median pVO2 of 1624 mL/min versus 1233 mL/min (p < 0.035), although the statistical difference was lost using indexed pVO2 (29 mL/min/kg [interquartile range 24.8–35.8] vs. 25.7 mL/kg/min [interquartile range 23.4–32], p = ns).
Association between additional ventricular chamber and clinical outcomes
The additional ventricular chamber was smaller than 20 mL/m2 in most patients. A larger additional ventricular chamber (indexed end-diastolic volume >20 mL/m2) was more frequently associated with functional single right ventricle rather than functional single left ventricle (43% vs. 3%, p < 0.01, Table 1). While the presence and size of the additional ventricular chamber did not affect the late clinical outcomes (Table 2), patients with a larger additional ventricular chamber showed a significantly higher need for post-operative extracorporeal membrane oxygenation support (p < 0.01), and a trend towards longer hospital stay after Fontan procedure (p = 0.069). No differences could be outlined in cardiac MRI or cardiopulmonary exercise test findings depending on additional ventricular chamber size (Table 2).
Table 2. Impact of AVC size on clinical outcomes and CMRI and CPET metrics
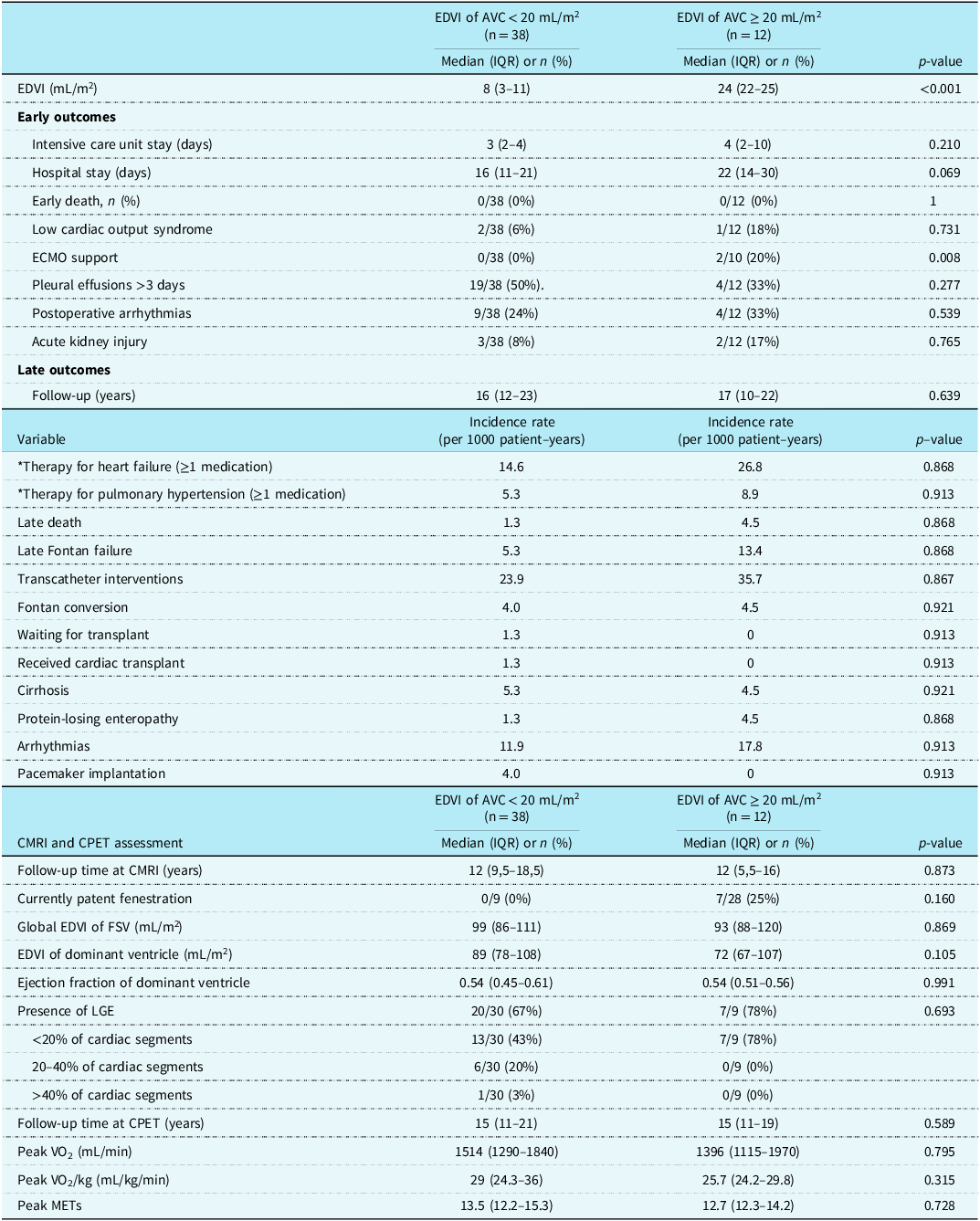
AVC = additional ventricular chamber; CMRI = cardiac MRI; CPET = cardiopulmonary exercise test; EDVI = indexed end-diastolic volume; FSLV = functional single left ventricle; FSRV = functional single right ventricle; IQR = interquartile range; LGE = late gadolinium enhancement; MET = metabolic equivalents. *Information regarding home therapy was not available in five patients.
To assess the haemodynamic role of an additional ventricular chamber that could potentially contribute to the systemic cardiac output, we performed a sub-analysis stratifying patients according to the presence/absence of a ventricular septal defect and the presence/absence of aortic atresia. Twenty patients (20/50, 40%) had an associated ventricular septal defect; cardiac MRI and cardiopulmonary exercise test findings were not influenced by the presence of a ventricular septal defect, except for pVO2/kg, which was higher in patients without a ventricular septal defect (30.9 [interquartile range 24.9–35.5] vs. 25.1 [interquartile range 23.1–26.3], p = 0.017, Table S5). In patients with aortic atresia (8/50, 16%), a higher severity of dominant ventricle dilatation, systolic dysfunction, and foci of late gadolinium enhancement was documented at cardiac MRI assessment, when compared with patients without aortic atresia (Table S6). Conversely, cardiopulmonary exercise test parameters were similar between the two groups.
Propensity score inverse of treatment weight model results
A suitable covariate balancing was evidenced for the dominant ventricle and additional ventricular chamber size (Figure 2). The propensity score-adjusted model results confirmed that in functional single left ventricle, there was a significant improvement in functional capacity at cardiopulmonary exercise test, with an expected increase of 2.05 in metabolic equivalent and an expected improvement of 286 in pVO2 (Table S7). Moreover, the propensity score analysis evidenced that a larger additional ventricular chamber (indexed end-diastolic volume >20 mL/m2) was associated with an expected increase in the hospital length of stay of 17 days (Table S7). Among the endpoints reported in Table S7, the functional capacity at cardiopulmonary exercise test is the only normally distributed variable according to the Shapiro–Wilk test.

Figure 2. Standard mean differences across groups before and after the propensity score adjustment for functional single right ventricle versus functional single left ventricle (A) and additional ventricular chamber size <20 mL/m2 versus ≥20 ml/m2 (B).
Lastly, surgical history did not significantly impact ventricular function and early or late outcomes in the different groups (functional single left ventricle vs. functional single right ventricle, additional ventricular chamber <20 vs. >20 mL/m2, Figure 2).
Discussion
Soon after its application, the Fontan operation was expanded to treat all types of functional single ventricle, even those where an additional ventricular chamber was deemed too small to provide adequate output. Reference Schilling, Dalziel and Nunn7,Reference d’Udekem, Iyengar and Galati8 This heterogeneous population can display significantly diverging long-term outcomes, depending on several risk factors that have been delineated through the years. Reference McGuirk, Winlaw and Langley24–Reference Kogon, Plattner, Leong, Simsic, Kirshbom and Kanter26 Great interest has been recently raised in the role of anatomical factors, such as the morphology of the functional single ventricle11 and the presence of an additional ventricular chamber, Reference Rossi, Frigo and Reffo14 since they may affect the long-term performance of the Fontan circulation.
In this study, we specifically addressed this topic considering the impact of functional single ventricle dominance and additional ventricular chamber quantified at cardiac MRI, on early and late clinical outcomes of Fontan palliation, as well as ventricular function measured on cardiac MRI and functional capacity on cardiopulmonary exercise test. In our cohort of 50 Fontan patients, functional single left ventricle was associated with lower need for heart failure-related medical therapy, a better ejection fraction, and lower prevalence and severity of ventricular fibrosis. This translated into a better functional capacity at cardiopulmonary exercise test, both in terms of pVO2 and metabolic equivalents (Table 1).
Several authors have previously reported worse outcomes for patients with an anatomical functional single right ventricle during the Fontan pathway. Reference d’Udekem, Iyengar and Galati8,Reference Erikssen, Aboulhosn and Lin27–Reference West, Maul, Feingold and Morell29 Functional single right ventricle has been shown to have a lower ejection fraction and reduced myocardial functional reserve when compared to functional single left ventricle, possibly related to an unsatisfactory functional adaptation to pressure and volume overload. Reference Ponzoni, Azzolina and Vedovelli11 A plausible explanation is that when the right ventricle (naturally facing lower pulmonary resistances) is reconnected to the systemic circulation, it may gradually remodel, resulting in lower power output and impaired myocardial deformation indexes when compared to functional single left ventricle physiology. Reference Sundareswaran, Kanter and Kitajima30,Reference Shiraga, Ozcelik and Harris31 Our findings suggest that the post-operative course in patients with functional single right ventricle may be characterised by a significantly higher incidence of low cardiac output syndrome, although other common morbidities were equally balanced between the two groups (Table 1). Similarly, several authors have reported that a relatively poor outcome after the modified Fontan operation can be expected in patients with functional single right ventricle, as a result of basic anatomic and haemodynamic problems. Reference Matsuda, Kawashima and Kishimoto32 This occurs especially in the early postoperative course, where right morphology was an independent risk factor for prolonged respiratory and inotropic support, as well as hospitalisation times. Reference McGuirk, Winlaw and Langley24,Reference Ovroutski, Sohn and Barikbin33 More recently, Pollak et al. Reference Pollak, Abarbanel, Salem, Serraf and Mishaly34 found that in a series of 98 Fontan patients (with extracardiac conduit), patients with a functional single right ventricle more frequently developed early transient signs of suboptimal haemodynamics, necessitating intensified cardiovascular support, prolonged mechanical ventilation, and longer hospitalisation. Importantly, in our cohort, functional single right ventricle patients presented with heterotaxy syndrome more frequently than functional single left ventricle, which can complicate the surgical rerouting of the inferior vena cava drainage into the pulmonary artery at the time of Fontan operation. Given the small number of patients with heterotaxy, we could not evaluate the specific impact of this variable on the development of early postoperative adverse outcomes.
Also, we found that patients with functional single right ventricle were more often requiring medical therapy for heart failure. This may imply a progressive deterioration of Fontan circulation performance with time. The generally more complex pre-Fontan staging course in functional single right ventricle, often requiring neonatal palliation and circulatory arrest for arch reconstruction/augmentation, has been associated with coronary perfusion abnormalities, Reference Kaneko, Khoo, Smallhorn and Tham35–Reference Ohye, Devaney, Hirsch and Bove37 whose long-term sequelae are unknown. Accordingly, cardiac MRI revealed a greater incidence of late gadolinium enhancement-positive areas in functional single right ventricle patients— in 94% (16/17) of patients. However, late gadolinium enhancement positivity was limited to <20% of cardiac segments in 70% of these patients, which may represent a late scar of the ventriculotomy for the Sano conduit. The impact of akinetic and dyskinetic myocardial segments on global function in single ventricle patients is still under investigation. Reference Sundareswaran, Kanter and Kitajima30,Reference Schwartz, Lu and Ohye36 Despite the relatively small number of patients, our propensity score inverse of treatment weight model did not identify any significant impact of the surgical history on ventricular function and early or late outcomes in the functional single left ventricle versus functional single right ventricle groups. Moreover, the types of surgical technique for Fontan completion (atrio-pulmonary vs. lateral tunnel vs. extracardiac conduit) are known to influence Fontan circuit haemodynamics and, potentially, its efficiency. Reference Mondésert, Marcotte and Mongeon4–Reference Padalino, Ponzoni and Castaldi6 However, the incidence rate of Fontan conversion, which reflects the failure of Fontan circulation inherent to its surgical construction, was balanced between groups. A specifically designed study to evaluate the impact of the type of neonatal palliation and Fontan type on the long-term fate of ventricular function and exercise capacity is required to address this topic.
Pulmonary artery architecture and physiology are well-recognized determinants of Fontan circuit performance. Reference Uemura, Yagihara and Kawashima12–Reference Rossi, Frigo and Reffo14 In the present cohort, only two patients (one functional single right ventricle and one functional single left ventricle) required interventional procedures for clinically significant pulmonary artery stenosis, while six patients are currently assuming medications for pulmonary hypertension. Although pulmonary vascular abnormalities may impact cardiopulmonary exercise test outcomes in Fontan patients, the observed low rates of these complications and their balanced distribution among the study groups is supposed to minimise this potential source of bias.
The role of the additional ventricular chamber in patients with functional single ventricle is still unclear. Reference Rossi, Frigo and Reffo14,Reference Kurotobi, Sano and Naito38–Reference Fogel, Weinberg and Gupta43 It has been speculated that through ventricular-ventricular interactions, a large additional ventricular chamber may impair the function of the primary ventricle. Reference Kurotobi, Sano and Naito38–Reference Wisler, Khoury and Kimball40,Reference Fogel, Weinberg and Gupta43 Furthermore, the presence of an additional ventricular chamber may alter intracardiac flow dynamics, potentially creating conflicting blood flow vortexes and turbulence that can deteriorate cardiac output or affect ventricular relaxation causing impairment of diastolic function. Reference Kamphuis, Roest, Westenberg and Elbaz42 Kurotobi et al showed that a larger additional ventricular chamber was associated with impaired regional shortening, asynchronous contraction, and greater end-diastolic pressure in the primary ventricle. Reference Kurotobi, Sano and Naito38 Fogel used tagged cardiac MRI to demonstrate that in patients with a hypoplastic right ventricle, left ventricular strain, radial motion, and twisting were abnormal. Reference Fogel, Weinberg and Gupta43 In contrast, Wisler and coworkers did not find a consistent relationship between the size of the hypoplastic left ventricle and echocardiographic measurements of global right ventricular function in patients with hypoplastic left heart syndrome. Reference Wisler, Khoury and Kimball40 Although these studies have cumulatively shown that the additional ventricular chamber can affect the regional functioning of the primary ventricle, the consequences of such ventricular–ventricular interactions on global cardiovascular performance have not been analysed. Interestingly, Prakash et al. demonstrated that exercise capacity was more preserved in subjects with a larger, and less hypertrophied secondary ventricle, which was able to make a greater contribution to the stroke volume. Reference Prakash, Travison and Fogel39 These data strongly challenge the hypothesis that a larger additional ventricular chamber alters the function of the primary ventricle in the Fontan circulation. In contrast, they support the intuitive notion that an additional ventricular chamber may contribute positively to the cardiac output by providing an additional stroke volume, or possibly decreasing the end-diastolic pressures, optimising the trans-pulmonary gradients.
In our series, the presence of an additional ventricular chamber was not a convincing predictor of any clinical advantage or disadvantage in the long term. Our findings parallel the recent report of Marathe and colleagues, Reference Marathe, Zannino and Shi41 who showed that freedom from Fontan failure was similar between patients with one versus two ventricles. Since we mostly analysed patients with an additional ventricular chamber not amenable to biventricular repair, it is important to underline that our results should not be intended for guiding the therapeutic decision-making between Fontan operation and biventricular repair. Conversely, our findings suggest that long-term ventricular–ventricular interactions and Fontan performance seem not to be influenced by the presence of a larger rudimentary ventricular chamber.
On the other hand, we found that the early post-operative course can be negatively influenced by the presence of an additional ventricular chamber >20 mL/m2, with higher need for post-operative extracorporeal membrane oxygenation support (p < 0.01, Table 2). It is of note that in this series, a large additional ventricular chamber has been more frequently associated with a functional single right ventricle. Thus, the independent role of the additional ventricular chamber on early post-operative haemodynamics may be masked by the strong association with functional single right ventricle. Interestingly, Wang et al. Reference Wang, Kelle and Hyun44 have investigated the impact of the left ventricular remnant on systemic functional single right ventricle myocardial deformation in hypoplastic left heart syndrome after the Fontan procedure, by means of cardiac MRI. They categorised the left residual chamber as absent/slit-like or lobular/miniaturized. Among 48 cardiac MRI scans, they demonstrated that larger left ventricular remnants with globular/miniaturized morphology negatively affected the global longitudinal and radial strain indices of the systemic functional single right ventricle.
Based on these findings, we infer that patients with a large additional ventricular chamber with functional single right ventricle should require closer monitoring postoperatively and in the long term, due to the detrimental association of ventricular morphology not intended to sustain the systemic afterload and lack of a well-defined contractile role of the additional ventricular chamber. Targeted clinical vigilance and early adoption of heart failure medications might contribute to preserving the functional capacity in this delicate population.
Limitations
There are some limitations in this study. Other than the retrospective nature and the relatively small sample size, main limitations are related to the fact that our Fontan cohort is quite heterogeneous in the duration of follow-up, operative characteristics, anatomical variants, associated pathologies, and evolution of surgical techniques in a relatively long cohort period (1980–2019). We accounted for the different follow-up times between the study groups using exposure-adjusted incidence rates of late adverse events. However, modifications in surgical and medical management may have happened during the cohort study period, whose impact will need dedicated investigations. Moreover, it cannot be excluded that cardiac MRI and cardiopulmonary exercise test findings may evolve at a longer follow-up in the functional single right ventricle group. In addition, mean age at evaluation was different between patients with functional single left ventricle versus functional single right ventricle, thus representing a potential source of survivor bias.
In order to avoid an over-estimation of ventricular ejection fraction, patients with moderate or more atrioventricular valve regurgitation were excluded from the analysis. Reference Ponzoni, Azzolina, Vedovelli, Gregori, Vida and Padalino45 Thus, our results might not be fully translatable to the subset of Fontan population affected by significant atrioventricular valve dysfunction. The extent of late gadolinium enhancement was quantified in the dominant ventricular chamber only, while further investigations are required to define the role of late gadolinium enhancement in the additional ventricular chamber and its clinical and haemodynamic implications. Finally, since cardiopulmonary exercise test requires discrete patient collaboration and a minimal threshold of functional capacity to be performed, we might have selected a healthier cohort of patients than the more general Fontan population. Thus, particular caution should be adopted in generalising our findings to the whole Fontan population.
Conclusions
Our study confirms previous literature showing better clinical and functional outcomes in functional single left ventricle patients late after the completion of the Fontan circulation. In our cohort of 50 Fontan patients, cardiac MRI demonstrated that functional single left ventricle patients displayed a 5% higher ejection fraction and a lower rate of any late gadolinium enhancement-positive foci of myocardial fibrosis (50% vs. 94% of cases), as well as a better cardiopulmonary performance at cardiopulmonary exercise test than functional single right ventricle subjects. However, the clinical impact of these findings has yet to be determined. Our results suggest that the early postoperative course may be negatively influenced by functional single right ventricle morphology, while the role of an additional ventricular chamber larger than 20 mL/m2 in early post-operative outcomes requires further investigation. Conversely, a fairly developed additional ventricular chamber (but not amenable for biventricular repair) was not apparently related to any clinical advantage or disadvantage in the long term, although larger studies are awaited for the confirmation of this finding. The inferior follow-up in the functional single right ventricle population must dictate a proactive clinical surveillance since myocardial remodelling and functional capacity impairment might further progress in the very long term.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1047951124026581.
Acknowledgements
None.
Financial support
None.
Competing interests
None.
Ethical standard
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008, and has been approved by the hospital committee for clinical investigation (protocol 59005).







