Social media synopsis
Phase 3 randomised study evaluating safety of oral edoxaban as alternative anticoagulant for children with cardiac diseases who are at risk of thromboembolism.
Thromboembolism is a major complication in children with cardiac diseases, congenital or acquired. Children with cardiac diseases constitute a major proportion of thrombosis patients admitted to tertiary hospitals. Reference Checchia, Karamlou, Maruszewski, Ohye, Bronicki and Dodge-Khatami1–Reference Emani, Zurakowski, Baird, Pigula, Trenor and Emani6 Up to 50% of infants <6 months of age and 30% of older children with venous thromboembolism have underlying cardiac diseases. Reference Monagle7 The highest risk groups include children with shunt-dependent single ventricles, central lines, Fontan circuit, Kawasaki disease with coronary aneurysms, and cardiomyopathy. Reference Giglia, Massicotte and Tweddell2 The risk factors for thromboembolism include alteration in hemodynamics, prosthetic materials, surgically damaged blood vessels, intravenous catheters, and cardiopulmonary bypass.
Thromboembolism leads to significant morbidity and mortality in children with cardiac diseases. Reference Checchia, Karamlou, Maruszewski, Ohye, Bronicki and Dodge-Khatami1,Reference Giglia, Massicotte and Tweddell2,Reference Monagle7 In a large retrospective cohort study of children undergoing cardiovascular surgery, 20.8% of the children diagnosed with thromboembolism died as its consequence. Reference Giglia, Bulas and Sell8 In a more recent study among similar cohort of patients, thromboembolism was associated with
-
longer intensive care unit stay (additional 10 days) and hospital stay (additional 15.2 days),
-
higher odds of cardiac arrest (odds ratio: 4.9), catheter re-intervention (odds ratio: 3.3), and
-
re-operation (odds ratio: 2.5) and increased mortality (odds ratio: 5.1). Reference Manlhiot, Menjak and Brandao3
The use of anticoagulation prophylaxis in select subpopulations and settings has become the standard of care to prevent thromboembolism and associated complications. Reference Monagle, Chan and Goldenberg9 Advances in medical and cardiac surgical techniques have led to improved survival in this group of children; Reference Jacobs, He and Mayer10 thus, the overall use of anticoagulation has increased in recent decades.
The commonly used agents for long-term anticoagulation in children are low molecular weight heparins and vitamin K antagonists. Reference Giglia, Massicotte and Tweddell2,Reference Monagle, Chan and Goldenberg9 The important disadvantage of low molecular weight heparin is subcutaneous administration. Reference Bidlingmaier, Kenet and Kurnik11 Vitamin K antagonists have a delayed onset of action, narrow therapeutic index, unpredictable pharmacologic response, and multiple food and drug interactions, and therefore require frequent monitoring. Reference Monagle and Newall12 Monagle et al showed that 41% of all warfarin measurements were below the target range in a group of 54 patients over a period of 2 years. Reference Monagle, Cochrane and Roberts13 The frequent blood monitoring and complexity of management pose a substantial burden on children and their families. Reference Monagle, Chan and Goldenberg9,Reference Andrew, Marzinotto and Brooker14,Reference Newall, Monagle and Johnston15 In one quality of life assessment study, 62% of parents reported warfarin therapy to be a burden “now and then” or “always”. Reference Jones, Monagle, Manias, Bruce and Newall16 In addition, administration of vitamin K antagonists in young children is challenging due to a lack of commercially available liquid formulation. Therefore, there exists a need for safe, effective, and easily manageable oral antithrombotic agent for the treatment of paediatric cardiac patients at risk of thromboembolism.
Direct oral anticoagulants overcome many of the above limitations for children. Direct oral anticoagulants can be administered orally, are antithrombin independent, have a rapid onset and offset of action, few drug and food interactions, and predictable pharmacokinetics with no need of routine monitoring of anticoagulation activity. Reference Albisetti17 Recently, two phase 3 direct oral anticoagulant trials for paediatric venous thromboembolism have been published. Reference Male, Lensing and Palumbo18,Reference Brandao, Albisetti and Halton19 The rivaroxaban phase 3 study showed that rivaroxaban was as safe and effective as standard anticoagulants in children with venous thromboembolism. Reference Male, Lensing and Palumbo18 A prospective cohort study showed a favourable safety profile of dabigatran for secondary venous thromboembolism prevention in children 3 months to <18 years of age with persistent risk factor(s). Reference Brandao, Albisetti and Halton19 In addition, the phase 3 randomised controlled trial of dabigatran for a conventional course of venous thromboembolism treatment was recently published. Reference Halton, Brandao and Luciani20
Edoxaban is an oral, direct, specific inhibitor of activated factor X. Its pharmacokinetic properties include rapid peak plasma concentration, low cytochrome P450 metabolism, limited protein binding, and linear pharmacokinetics. These properties allow for once daily dosing, which is an important advantage in comparison with low molecular weight heparins and some direct oral anticoagulants. Reference Parasrampuria and Truitt21 After initial heparin, edoxaban therapy was non-inferior to warfarin in adults with venous thromboembolism, and adults treated with edoxaban had fewer major or clinically significant bleeding complications. Reference Buller, Decousus and Grosso22 The paediatric investigational plan of edoxaban started with a phase 1, open-label study administering a single dose of edoxaban to children who required anticoagulant therapy for venous thromboembolism and aimed to identify paediatric doses in children ages 0 to <18 years that approximate drug exposure observed in adults (NCT02303431). Following this, a phase 3, prospective, randomised clinical trial was started, which is evaluating the efficacy and safety of edoxaban compared with standard of care for the treatment of venous thromboembolism (NCT02798471). Reference Van Ommen, Albisetti and Chan23 This purpose of this manuscript is to describe the rationale and design of the DU176B-C-U313 study, which is a phase 3, prospective, randomised controlled trial comparing the safety of edoxaban with the standard of care treatment in children with cardiac diseases who require anticoagulation to prevent primary and secondary thromboembolic disease.
Materials and methods
Study objectives and hypothesis
The primary objective of this study is to compare the safety of edoxaban with standard of care in regard to the combination of major and clinically relevant non-major bleeding in the main treatment period. Major and clinically relevant non-major bleeding is defined per published International Society on Thrombosis and Haemostasis criteria. Reference Schulman and Kearon24 The main treatment period will consist of the duration from randomisation to month 3 visit, or to the date of last dose of study drug plus 3 days if study treatment is discontinued, whichever is earlier.
The secondary and exploratory objectives are summarised in Table 1.
Table 1. Secondary and exploratory objectives of the study and corresponding time points
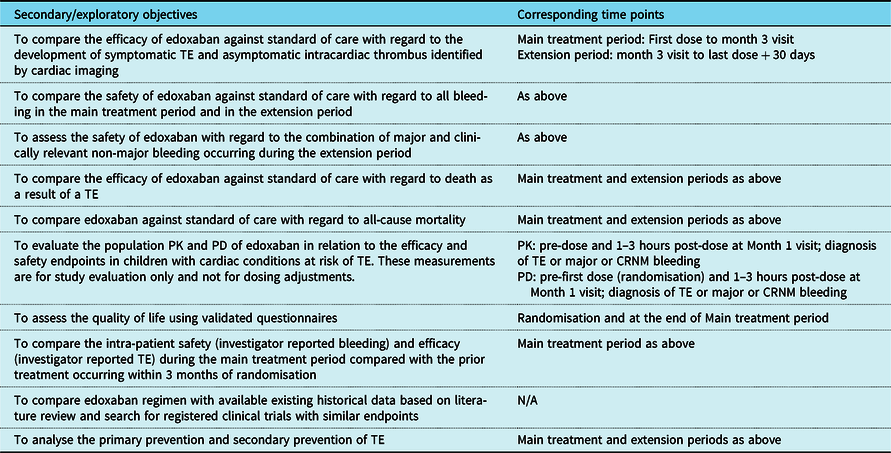
CRNM = clinically relevant non-major; PD = pharmacodynamics; PK = pharmacokinetics; TE = thromboembolism
The study hypothesis is that edoxaban is at least as safe as the current standard of care (low molecular weight heparins and/or vitamin K antagonists) with regard to the incidence of major and clinically relevant non-major bleeding in children with cardiac diseases at risk of thromboembolism.
Study design
This is a phase 3, multi-national, open-label, randomised, parallel-group, observational trial to evaluate and compare the safety and efficacy of edoxaban against standard of care. The trial is sponsored by Daichii Sankyo. The protocol was reviewed by institutional review boards at each participating centre. Eligible patients or their parents/caretakers provide informed consent before randomisation. The study is coordinated by a steering committee that provides clinical guidance on study implementation and study conduct. An independent data monitoring committee will monitor safety and other outcomes of the participating patients and make recommendations to the steering committee at intervals during the study. The adjudication of the safety and efficacy endpoints will be conducted by a blinded adjudication committee. The overall study design is shown in Figure 1.
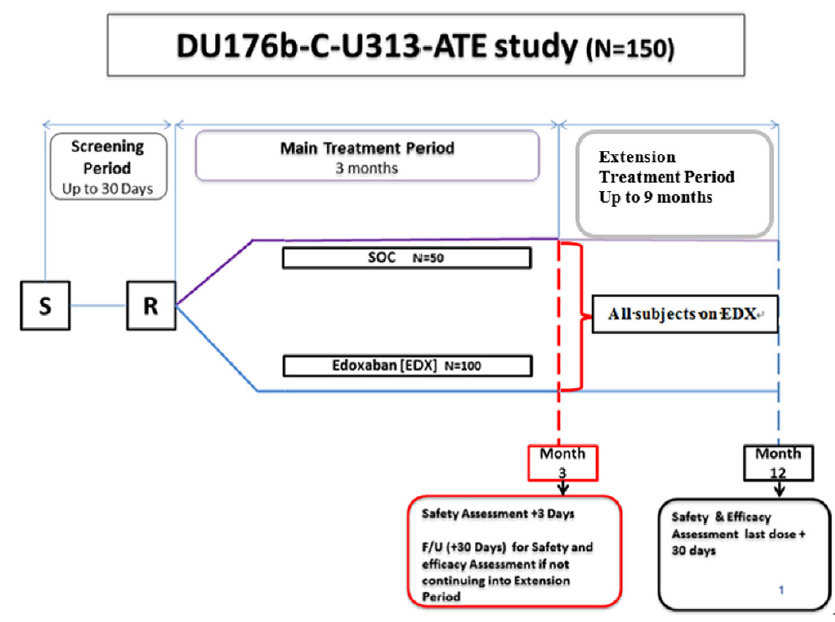
Figure 1. Overall study design: the figure outlines screening period of up to 30 days, main treatment period of 3 months from randomisation, followed by extension treatment period of up to 9 months from month 3 to month 12.
Note: S – screening; R – randomisation; SOC – standard of care; F/U – follow-up.
The inclusion and exclusion criteria are listed in Table 2. After assessment and confirmation of eligibility, patients will be randomised in a 2 to 1 ratio for edoxaban arm to standard of care arm, respectively. Randomisation will be stratified based on:
-
Underlying cardiac disease:
-
Kawasaki disease,
-
Previous Fontan operation,
-
Heart failure, and
-
Other (including post-surgical procedures other than the Fontan operation).
-
-
Patients with conditions other than Kawasaki disease will be further stratified based on the concomitant use of low-dose aspirin (1–5 mg/kg/day).
Table 2. Inclusion and exclusion criteria
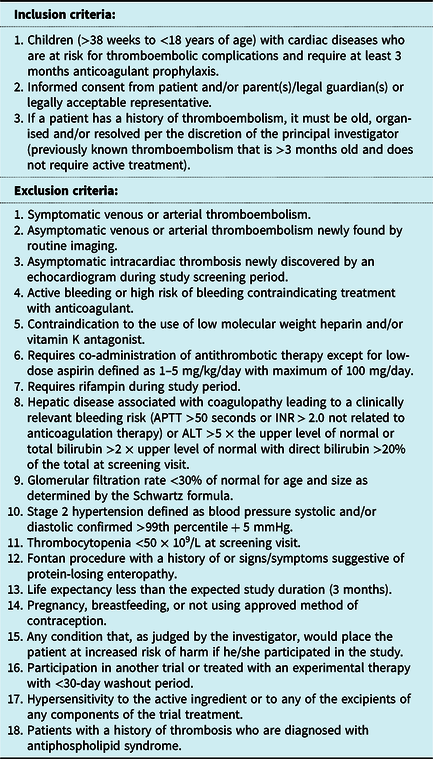
ALT = alanine transferase; APTT = activated partial thromboplastin time; INR = International Normalised Ratio
The patients will be recruited in four age cohorts: 12 to <18 years, 6 to <12 years, 2 to <6 years, and 6 months to <2 years. Patients from 1 to <18 years of age will be enrolled in the study as soon as the dosing regimen is established for each age cohort in the phase 1 single-dose edoxaban study. Patients <1 year of age will be admitted to the study after review by the independent data monitoring committee of safety data from 50% of patients in the 1 to <18 year age group who have completed the main treatment period of 3 months.
Treatment groups
The recommended dosing of edoxaban for the 12- to <18-, 6- to <12-, and 2- to <6-year-old patients is shown in Table 3. Dosing recommendations for patients <1 year of age will be provided as the phase 1 single-dose edoxaban study completes the pharmacokinetic exposure analysis for similar age cohorts (NCT02303431). Cohort 1 (12 to <18 years old) and Cohort 2 (6 to <12 years old) will receive tablets (15 and/or 30 mg strength), which may be crushed and served with applesauce or water. Children <6 years of age or those with a swallowing issue will receive edoxaban granules for oral suspension. Edoxaban doses will be reduced for patients with moderate renal impairment (defined as glomerular filtration rate of ≥30–≤50% of normal as determined by the Schwartz formula) and for patients requiring concomitant administration of certain P-glycoprotein inhibitors.
Table 3. Edoxaban doses for 6- to <18-year-old children
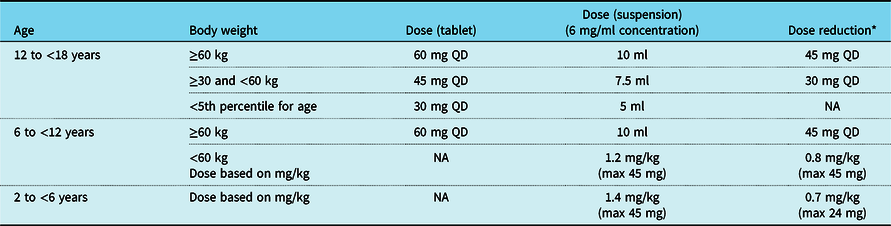
NA = not applicable; QD = once a day
* Conditions for dose reduction: concomitant administration of P-glycoprotein inhibitor and moderate renal impairment (defined as glomerular filtration rate of ≥30–≤50% of normal as determined by the Schwartz formula)
The standard of care arm consists of low molecular weight heparins or vitamin K antagonists according to the treating physician’s preferred regimen. It is the investigator’s discretion based on local practice and on the patient’s underlying cardiac disease to consider if heparin bridging is necessary when initiating vitamin K antagonists post randomisation until an International Normalised Ratio between 2.0 and 3.0 is reached.
All patients randomised to standard of care arm will be treated with standard of care regimen during the main treatment period (randomisation to month 3) and then offered edoxaban for the extension period (month 3 to month 12).
Study endpoints
All safety and efficacy endpoints described will be adjudicated in a blinded manner by the clinical events committee. An independent data monitoring committee will monitor safety throughout the duration of the study. The primary safety endpoint is a combination of major bleeding, as per International Society on Thrombosis and Haemostasis definition, and clinically relevant non-major bleeding occurring during the main treatment period.
Secondary endpoints are listed in Table 1.
Duration of study and follow-up
The study is divided into main treatment and extension periods. In the main treatment period, patients will be treated with edoxaban or standard of care as per their randomisation. During the extension period, all patients will be offered treatment with edoxaban.
All patients will undergo safety and efficacy evaluations at the end of main treatment period at month 3 with a 30-day follow-up. Along with review of symptomatic endpoints such as bleeding and symptomatic thromboembolism, safety evaluations will include complete blood count, liver function tests, creatinine, and estimated glomerular filtration rate measurements. Patients who discontinue treatment prior to month 3 will continue with the scheduled visits up to month 3 with the 30-day follow-up. Patients who participate in the extension period will undergo safety and efficacy endpoint evaluations (described above) at months 6, 9, and 12, and 30 days after cessation of treatment if treatment up to 12 months is required.
A follow-up echocardiogram will be taken at month 3 to ascertain asymptomatic intracardiac or coronary artery thrombosis. A month 3 echocardiogram will be optional if the patient experiences symptomatic thrombosis documented by an image, such as standard or CT angiogram, prior to this time. In the extension period after month 3, any symptomatic event will be documented, and a recommended image captured to support the event. A month 12 echocardiogram will be optional.
Sample size and statistical analysis
This study is not powered to demonstrate statistically significant differences in the rate of endpoint occurrences between edoxaban and standard of care. An adequately powered study would be unachievable due to the very large sample size required and the enormous challenge inherent in enrolling these children. To estimate a reasonable sample size, data were extrapolated from the adult Hokusai-VTE study (i.e., 8.5 and 10.3% in edoxaban and standard of care arms, respectively) as there is no pre-existing data for edoxaban in children. Reference Buller, Decousus and Grosso22 Assuming that the major and clinically relevant non-major bleeding event rates in the paediatric cohort will be similar, the 95% confidence interval around the point estimate of the risk difference between edoxaban and standard of care is −11.8 to 8.2% with 100 patients in edoxaban arm and 50 patients in standard of care arm. There is no intent to compare this paediatric study results with the adult Hokusai-VTE trial data.
The primary safety analysis will be performed according to the actual treatment received and will include composite of major and clinically relevant non-major bleeding occurring during the main treatment period. The time to major or clinically relevant non-major bleeding will be compared between treatment groups using the Cox proportional hazards regression model including treatment group, concomitant usage of aspirin, and underlying disease (Kawasaki disease, previous Fontan operation, heart failure, or other) as covariates. Hazard ratio between edoxaban and standard of care treatment groups will be calculated with corresponding 95% confidence intervals. The incidence, annualised event rate, and rate difference between edoxaban and standard of care treatment groups will also be calculated and compared with event rates in similar paediatric populations/indications from prior literature. All secondary safety analyses will also be performed based on the actual treatment received. All efficacy analyses will be based on the treatment group assigned at randomisation (modified intention to treat).
There is no formal interim analysis planned. However, an interim assessment by the independent data monitoring committee of safety endpoints of the study will take place after the first 50 patients in the edoxaban treatment group and 25 patients in the standard of care arm from 1 to <18 years of age complete the first 3 months of treatment. This assessment is pre-requisite to opening enrolment of patients <1 year of age on the trial.
Plasma concentration and biomarker data will be summarised by age, dose, and time point using descriptive statistics. The plasma concentration data will be pooled with data from other studies for a population pharmacokinetic analysis using non-linear mixed effects modelling. Exposure–response relationships will be evaluated for the safety and efficacy endpoints through a model-based approach, if data allow.
Discussion
Large randomised controlled trials are very difficult to perform in children with thrombosis or at risk of thrombosis secondary to multiple challenges. First, despite increasing incidence, thromboembolism remains an infrequent condition in children overall. Reference Raffini, Huang, Witmer and Feudtner25–Reference van Ommen, Heijboer, Buller, Hirasing, Heijmans and Peters27 Second, children who experience thromboembolism or are at risk of thromboembolism are sick with multiple underlying diseases and many concomitant medications. As a result, a large proportion of eligible patients are excluded from studies due to increased bleeding risk. Third, it is difficult for parents to give permission for study participation, especially when their children are young and very sick. These challenges were reflected in the early closure of some trials due to slow recruitment. Reference Monagle, Cochrane and Roberts13,Reference Massicotte, Julian and Gent28–Reference Mitchell, Andrew and Hanna30 In the paediatric anticoagulant field, few phase 3 trials have successfully completed planned enrolment; recent exceptions include the investigator-led NIH-sponsored Kids-DOTT trial, Reference Goldenberg, Abshire and Blatchford31 EINSTEIN-Junior, Reference Male, Lensing and Palumbo18 and DIVERSITY. Reference Halton, Brandao and Luciani20 A few additional industry-sponsored phase 3 studies of direct oral anticoagulants are ongoing, but few have been powered to demonstrate non-inferior or superior safety and efficacy relative to the standard of care. Reference Male, Thom and O’Brien32
The paediatric direct oral anticoagulant trials, including this study, are the outcome of collaboration between pharmaceutical companies and academic experts in the fields of paediatric thrombosis and paediatric cardiac care. To develop high-quality trials to increase the evidence of antithrombotic treatment in children with thromboembolism or at risk of thromboembolism, collaboration between industry and a multi-disciplinary group of thought leaders is needed, from program development and specific trial design to trial oversight, analysis, and reporting. Reference Goldenberg, Spyropoulos and Halperin33 Pharmaceutical companies benefit from the knowledge of the academic experts in the domains of paediatric thrombosis and paediatric cardiac care for the development of their paediatric investigational plan, and in return, the pharmaceutical companies provide logistics and funding for the large, international trials. Recently, the International Society on Thrombosis and Haemostasis paediatric subcommittee has established a task force for drug development in paediatric thrombosis to provide expert input into the development of industry-initiated studies. Reference Male, Monagle, Chan and Young34
The strengths of the ENNOBLE-ATE trial design include a more inclusive bleeding outcome, two-level stratification for randomisation, observational data with edoxaban for all patients, and objective quality of life assessments. As edoxaban is being evaluated for prophylaxis, it is appropriate to employ an inclusive definition for the outcomes of clinically important bleeding events. In a given target population, thromboembolism prevention (in comparison to treatment) involves a lower a priori risk of new thromboembolism events. Hence, from a net clinical benefit perspective, a higher rate (and/or greater severity) of bleeds is generally found to be acceptable for treatment of thromboembolism in comparison with prevention of thromboembolism. Hence, it is important to measure safety broadly by including all clinically relevant bleeding events, in addition to major bleeding, when evaluating net clinical benefit of the employed strategy of prevention of thromboembolism.
Two-level stratification is employed in this study to address the a priori differential risk of thromboembolism and bleeding based on underlying cardiac disease and the use of concomitant aspirin. Stratified randomisation assures that within each stratum of interest, a balance in randomised arms of treatment will be achieved. In children with Kawasaki disease, the incidence of coronary artery aneurysms is 5% with upfront treatment with immunoglobulin and high dose aspirin (previously 20–25%). Reference McCrindle, Rowley and Newburger35 Coronary artery aneurysms pre-dispose children to significant risk of coronary arterial thromboembolism, myocardial infarction, or sudden death. The risk of thromboembolism following the Fontan operation is reported to be 17–33%, Reference Balling, Vogt, Kaemmerer, Eicken, Meisner and Hess36 while the prevalence of thromboembolism is 14% among children with heart failure secondary to cardiomyopathy. Reference McCrindle, Karamlou and Wong37,Reference Gunthard, Stocker and Bolz38 The second level of stratification, based on the use of concomitant aspirin, is employed to address the suspected a priori differential risk of bleeding associated with anticoagulant monotherapy versus a combination of anticoagulation plus antiplatelet therapy. A similar study assessing another direct oral anticoagulant for patients with cardiac diseases has employed stratification based on age and type of cardiac disease, but not concomitant use of aspirin. Reference Payne, Burns and Glatz39 Another recent large randomised controlled trial in the field of paediatric anticoagulation, the Kids-DOTT trial on duration of anticoagulation for thromboembolism treatment, has also used stratified randomisation—specifically, based on age and thrombus location. Reference Goldenberg, Triputti and Crowther40
This study will generate observational data with edoxaban on all enrolled patients. While in the main treatment period, patients will be treated with edoxaban or standard of care as per their randomisation, all patients will be offered treatment with edoxaban in the extension period. This design feature distinguishes the ENNOBLE-ATE study from another paediatric direct oral anticoagulant trial in this population, Reference Payne, Burns and Glatz39 as it will provide comparative short-term data, as well as longer-term prophylaxis data in those electing to continue in the extension study.
An important exploratory objective of this study is quality of life assessment. Once-daily oral anticoagulant prophylaxis without need for frequent blood monitoring is an ideal solution against issues with current standard of care that pose significant burden for children and families. Quality of life is a key design element for current and future trials of antithrombotic therapy in children, particularly those trials that address non-hospitalised settings and/or chronic use. This issue has received too little attention in historical trials designs, but is particularly important in trials of oral agents that offer potential pragmatic benefits relative to parental agents used in the standard of care, even when safety and/or efficacy of the newer oral agents may be only non-inferior to parental agents used in the standard of care.
This study is not powered with intention to draw conclusions based on statistically significant differences between edoxaban and standard of care. An adequately powered study would not be feasible due to the significantly large sample size required in such a specialised paediatric population, as discussed above. However, the sample size does allow for appropriate 95% confidence interval around the point estimate of the risk difference between edoxaban and standard of care, based on observed bleeding rates in the adult Hokusai-VTE study. Reference Buller, Decousus and Grosso22
One of the limitations of this trial is the open design. However, investigator-reported outcome events (potential safety and efficacy endpoints) will be reviewed by an independent panel of clinical endpoint committee adjudicators who are blinded to treatment arm. In children, it is unethical to use placebo subcutaneous injections (for masking in regard to the use of low molecular weight heparins) or to perform false international normalised ratio tests (for masking in regard to the use of warfarin) in patients randomised to edoxaban.
In summary, randomised controlled anticoagulation trials are challenging in children. The edoxaban phase 3 study in children with cardiac diseases will adequately assess safety of edoxaban in comparison with current standard of care as a primary objective, as well as efficacy as a secondary objective. If the primary hypothesis regarding safety is supported by the findings of the trial, this study will provide key evidence towards a valuable alternative for anticoagulant thromboprophylaxis in children with cardiac diseases who are at risk of thromboembolism.
Acknowledgements
We would like to acknowledge and thank all the participating investigators, coordinators, patients, and parents.
Financial support
This trial is funded by Daiichi Sankyo Inc. The drafting and revision of this manuscript, however, was completed by the listed authors only.
Conflicts of interest
Any conflicts of interest by the authors have been disclosed to the journal.
Ethical standards
The authors state that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.







