Duchenne–Becker muscular dystrophinopathies are the most common inherited muscular diseases of childhood, with an estimated prevalence in the United States of America around 1.5 per 10,000 boys. Reference Romitti, Zhu and Puzhankara1 Duchenne muscular dystrophy affects between 3600 and 6000 live male births per year. Reference Romitti, Zhu and Puzhankara1–Reference Mercuri, Bönnemann and Muntoni3 Mutations in the Duchenne muscular dystrophy gene (Xp21.2 locus) lead to the absence or insufficient function of the dystrophin protein, causing progressive muscle damage and degeneration, resulting in muscle weakness, motor retardation, loss of ambulation, respiratory failure, and cardiomyopathy. Reference Wein, Alfano and Flanigan4,Reference Birnkrant, Bushby and Bann5 The age of onset, the severity of the symptoms, and the speed of progression is variable. Therefore, the diagnosis and prognosis of Duchenne muscular dystrophy-related cardiomyopathy is challenging and unreliable, Reference Mercuri and Muntoni6 since patients with very limited activity do not develop symptoms until severe ventricular dysfunction occurs.
The prognosis of dystrophinopathies, and especially Duchenne muscular dystrophy, has improved in recent years thanks to the establishment of an early multidisciplinary care. Early treatment with corticosteroids and adequate respiratory, nutritional, physiotherapeutic, and orthopaedic management have allowed to slow the progression rate of the disease. Reference McDonald, Henricson and Abresch7 In this context, cardiac involvement is a complication that takes on special prominence, becoming one of the main causes of morbidity and mortality. Given the physical limitation of this type of patient, cardiac deterioration in dystrophinopathies tends to be asymptomatic, so its diagnosis is based on periodic complementary tests. Reference Nascimento Osorio, Medina Cantillo, Camacho Salas, Madruga Garrido and Vilchez Padilla8,Reference Power, O’Grady, Hornung, Jefferies, Gusso and Hofman9 Late cardiac evaluation, once heart dysfunction or clinical manifestations are evident, leads to late treatment and poor outcomes. Reference Birnkrant, Bushby and Bann10,Reference Bushby, Finkel and Birnkrant11 Therefore, early identification of cardiac muscle deterioration is crucial to start heart failure treatments, that reverse remodelling and improve prognosis. Reference Mercuri, Bönnemann and Muntoni3 Left ventricular dysfunction turns into dilated cardiomyopathy causing heart failure and arrhythmias. Reference McNally, Kaltman and Woodrow Benson12
Transthoracic echocardiogram continues to be the method of choice for the diagnosis and follow-up of dystrophinopathies associated with dilated cardiomyopathy Reference Schultheiss, Fairweather and Caforio13 given its wide availability and tolerability. However, conventional echocardiographic measures of LV function, such as left ventricular ejection fraction fail to reveal subclinical deterioration at early age. Reference Corrado, Lissoni and Beretta14 More recent alternative techniques are being investigated for early assessment, Reference Oreto, Vita and Mandraffino15 such as longitudinal deformation or strain. Reference Power, O’Grady, Hornung, Jefferies, Gusso and Hofman9 Several studies have reported decreased values of global longitudinal strain on patients with Duchenne muscular dystrophy, even though left ventricle ejection fraction was within the normal range. Reference Oreto, Vita and Mandraffino15–Reference Habib and Mohamed21
Being a progressive disease, the cardiac function of Duchenne patients deteriorates with age. These age effects are unknown and probably not linear. This study aims at characterising cardiac outcome measure (left ventricle ejection fraction and global longitudinal strain) as a continuous function of age in order to discover the most sensitive outcome for Duchenne patients, i.e. the one that provides an earlier detectable change or sign. Accordingly, the characterisation of age effects on each cardiac outcome will be a valuable tool for the assessment of disease progression.
Recent studies have tried to quantify age effects on global longitudinal strain, but they have used either dichotomisation with arbitrary age cut-off values Reference Oreto, Vita and Mandraffino15,Reference Hor, Taylor and Al-Khalidi27 or a linear assumption. Reference Amedro, Vincenti and de La Villeon16 In contrast, in this study age effects are assessed for both left ventricle ejection fraction and global longitudinal strain by using a continuous non-linear function, allowing more complex progression patterns.
Materials and methods
Study design and population
An observational, cross-sectional, case–control study was carried out from March to October 2020. Male Duchenne muscular dystrophy patients with classical phenotype and genetic confirmation tests were recruited as the cases. Healthy, age-matched, male subjects were enrolled as the controls. The study was approved by the Research Ethics Committee of the Autonomous Community of Aragon, Spain (CEICA). Informed consent was obtained from all subjects or their parents.
Echocardiogram
All studied individuals underwent an advanced transthoracic echocardiogram using a Siemens Acuson SC2000 (Siemens Healthcare, Erlangen, Germany) ultrasound device with a 4 MHz sector probe. The study involved 2D, Doppler, M-mode, and tissue Doppler assessment. Two studies were disregarded due to not enough image quality for speckle tracking assessment.
Echocardiographic measurements were performed according to the recommendations from American Society of Echocardiography and European Society of Cardiovascular Imaging. Reference Lang, Badano and Mor-Avi22 The parasternal long-axis view was used to assess the left ventricular end-diastolic diameter and it was reported as Z-score values using Haycock formula and Cantinotti’s nomograms. Reference Cantinotti, Scalese and Murzi23 The apical four-chamber view was used to assess left ventricle ejection fraction using the Simpson method, velocity of the lateral S´ wave of the mitral annulus (tissue Doppler), TEI index (tissue Doppler), and the study of diastole using the E/A and E/e' ratios.
Speckle tracking echocardiography
Myocardial strain was assessed by means of speckle-tracking echocardiographic. High resolution images (>65 frames/second) of the LV were recorded in the apical four chamber view. A posteriori analysis was performed using Velocity Vector Imaging 3.0 software to assess global longitudinal strain. Under electrocardiographic control, at least three cardiac cycles were analysed. Endocardial and epicardial borders delineated at the end of a diastole were used as initialisation for the cardiac motion assessment. Reference Geyer, Caracciolo and Abe24 The study was carried out based on six segments in the longitudinal deformation. Strain (%) was evaluated in each of the studies.
Statistical analysis
At the initial stage, exploratory data analysis was performed in order to visualise patterns on the cardiac function measurements and potential associations with covariates. Bayesian linear regression was the main tool for inference. A Bayesian paradigm was chosen because it allows the quantification of uncertainty in the parameters as well as with predictions. Reference Althouse, Below and Claggett25
An independent model was fitted to each of the outcomes: global longitudinal strain and left ventricle ejection fraction. A comparison between several models were performed including age effects and/or heart rate effects. In order to explore the potential non-linear contribution of age and heart rate effects to cardiac outcomes, a spline function (a smooth continuous curve) was considered instead of standard linear terms. Age effects are parameterised by means of a spline function because of its simplicity to characterise continuous and smooth contributions. The underlying assumption is that age effects may be different for each patient group, and therefore an interaction term is included, i.e. the smooth spline function is different for each group. The brms package in R was used to fit each regression model. Reference Bürkner26 Statistical inference was performed in terms of credible intervals for the posterior distribution of model parameters and predictions.
Results
Population
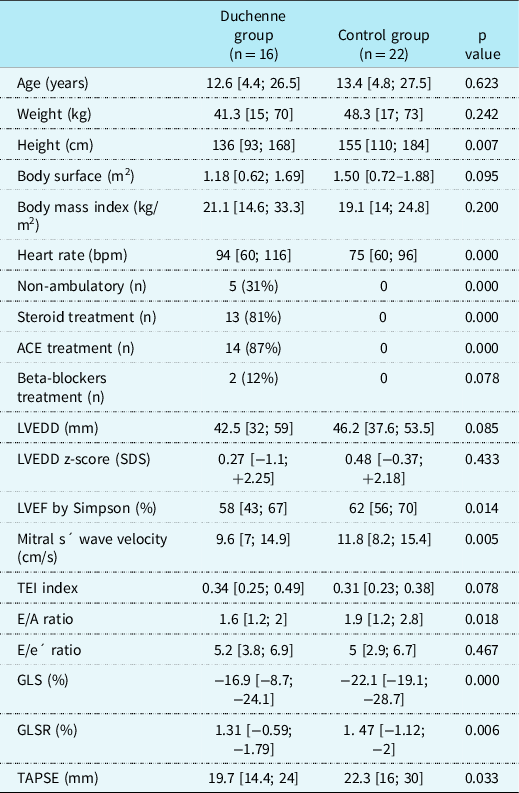
Sixteen Duchenne muscular dystrophy patients were recruited as the cases. Twenty-two healthy male subjects were enrolled as the controls. Statistical descriptors of anthropometric, clinical, and cardiac ultrasound variables are given in Table 1. As expected, the control subjects were taller than Duchenne patients. Five out of the 16 Duchenne patients could not walk. Duchenne patients showed higher heart rate and worsened values of most cardiac function parameters. All Duchenne patients older than ten were on treatment against heart failure; most of them received steroids.
Table 1. Anthropometric, clinical and cardiac ultrasound characteristics of Duchenne muscular dystrophy and control groups. Scalar variables are shown as mean/median and range (minimum; maximum). Nominal variables are shown as the number of patients (n) and percentage. T-Student, U Mann-Whitney and Chi-Square tests for comparison between groups. ACE: Angiotensin-converting enzyme. LVEDD: left ventricular end-diastolic diameter. GLS: global longitudinal strain. GLSR: global longitudinal strain rate. TAPSE: Tricuspid annular plane systolic excursion.
Exploratory data analysis
The panels along the main diagonal in Figure 1 show the distribution difference among groups for the main explanatory variables: age, heart rate, global longitudinal strain, and left ventricle ejection fraction. It is clearly shown that Duchenne patients have higher heart rate as well as worsened cardiac function measures, including global longitudinal strain and left ventricle ejection fraction. Interestingly though, the rest of the panels show scatter plots of the pairwise association between variables. For example, the panel at the third row and first column describes the age effects of the global longitudinal strain variable for each patient group: global longitudinal strain global longitudinal strain deteriorates for older Duchenne patients, while control subjects do not show that age effect. A similar phenomenon can be observed in the panel at the fourth row and first column, where the age effects of ejection fraction is shown. Duchenne patients show an ejection fraction worsening while the ejection fraction of control subjects does not show age effects.
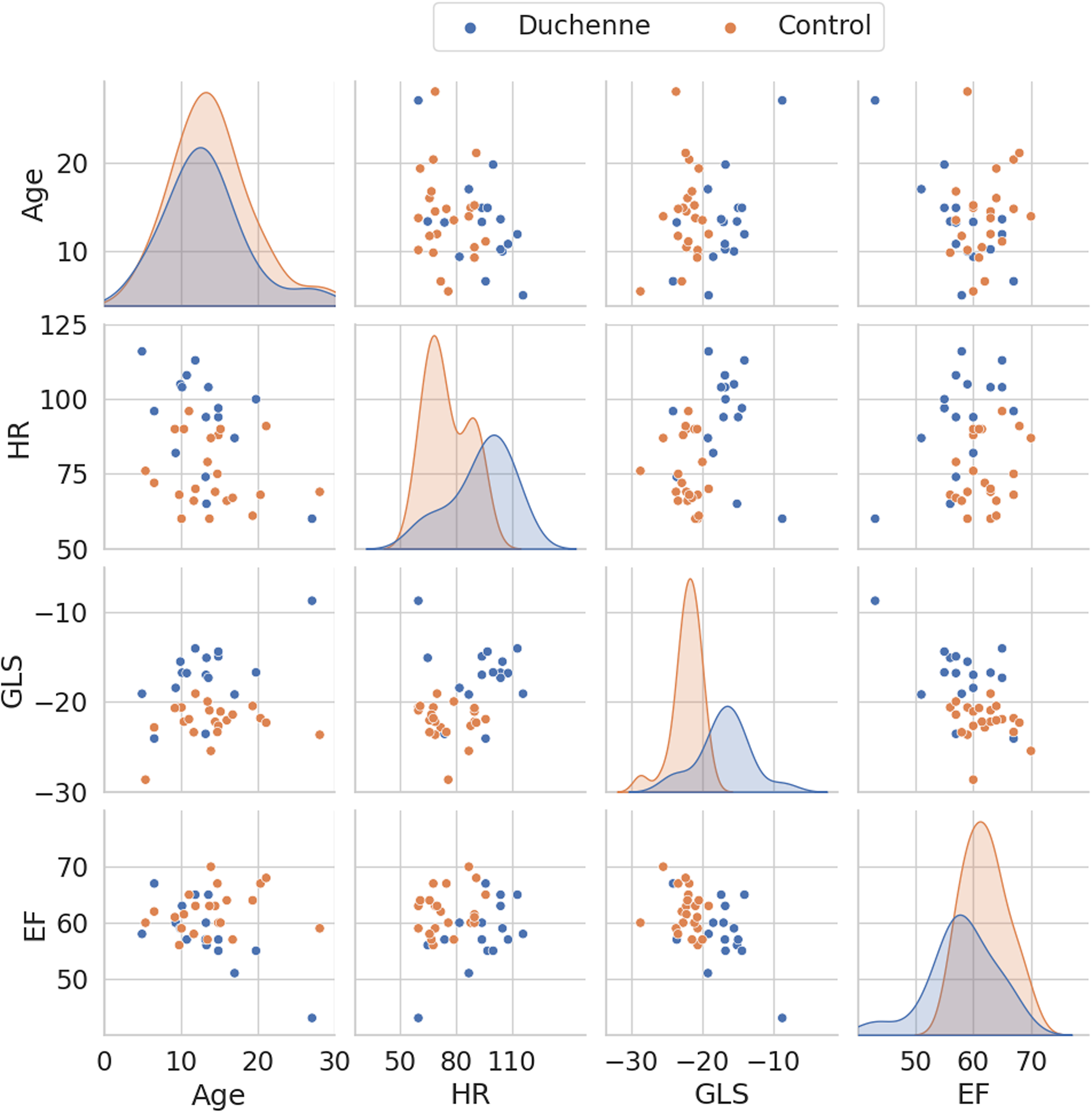
Figure 1. Pair plot illustrating the pairwise associations of the following variables: age, heart rate, global longitudinal strain and left ventricle ejection fraction. The panels along the main diagonal show the univariate distributions. The off-diagonal panels show the scatter plot of each variable pair. GLS: global longitudinal strain. HR: heart rate.
Statistical models
A set of Bayesian regression models was fitted on each cardiac function outcome in order to assess both age and heart rate effects. As an illustration, Figure 2 shows the age effects on left ventricle ejection fraction, global longitudinal strain, and heart rate, i.e. each regression model only included a single term accounting for age effects and parameterised with a spline function. As expected from progressive diseases, as the subjects become older, global longitudinal strain, and left ventricle ejection fraction get worse. Global longitudinal strain showed an approximately linear trend versus age with a rate of 0.37%/year, and the credible intervals for the average global longitudinal strain do not overlap for subjects older than 7 years. In contrast, the age effects on left ventricle ejection fraction are non-linear, including an initial plateau and a subsequent deterioration phase after around 13 years. It is worthy to note that the average prediction of left ventricle ejection fraction crosses the standard 55% cut-off value at an age of 16 years. It is also observed that Duchenne patients showed a faster heart rate, especially the younger patients. Note that the uncertainty in the average predictions is larger/smaller at the age intervals with less/more observations, as expected.
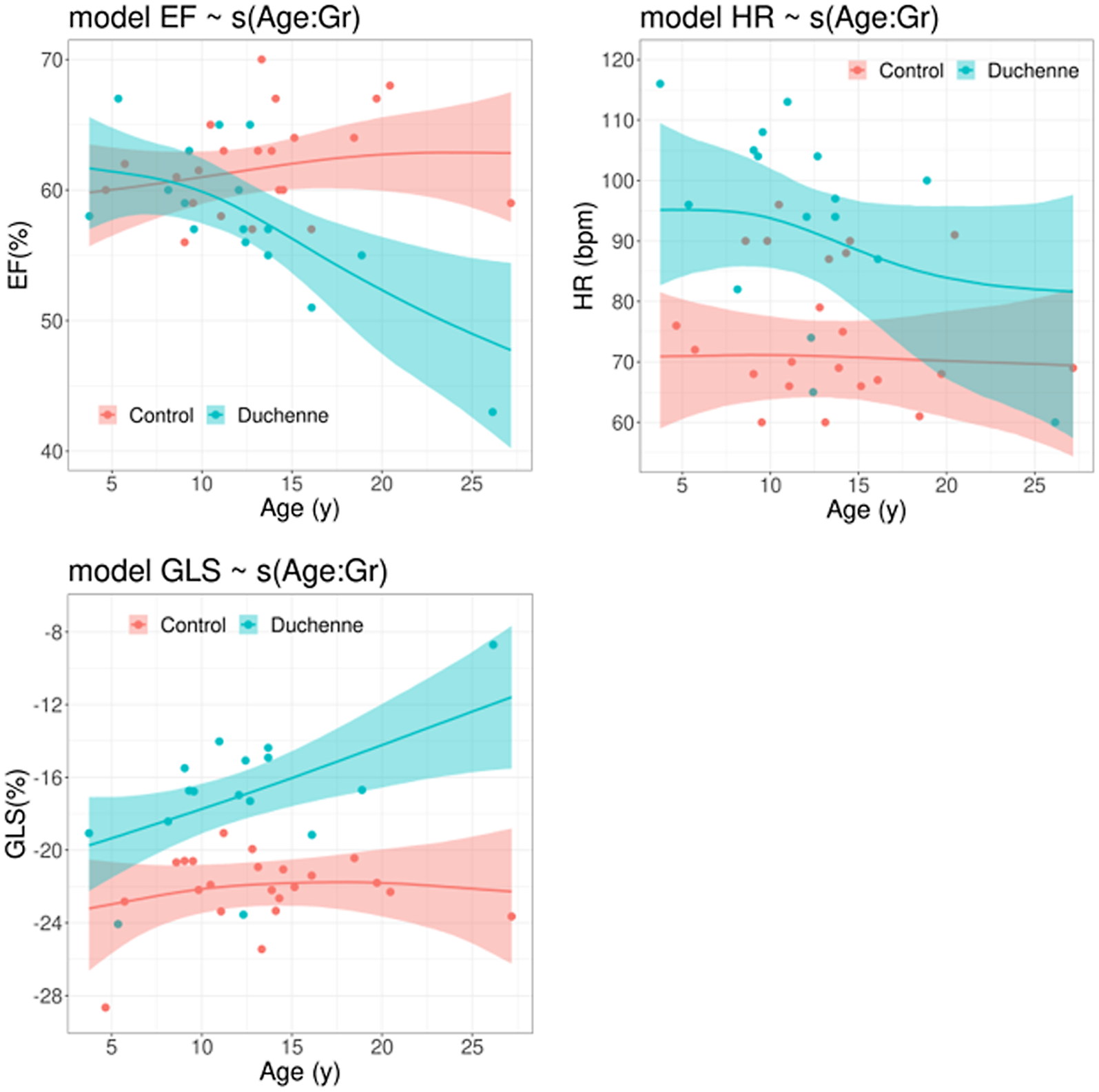
Figure 2. Posterior distribution of the predicted average for left ventricular ejection fraction (top left), global longitudinal strain (bottom left) and heart rate (bottom right) determined by age-effects. The solid line represents the predicted average and the coloured region the corresponding 95% credible interval for the average. GLS: global longitudinal strain. HR: heart rate. LVEF: left ventricular ejection fraction.
Similarly, Figure 3 shows heart rate effects on both cardiac results, left ventricle ejection fraction, and global longitudinal strain. Again, the group differences as a function of heart rate are more visible for global longitudinal strain than for left ventricle ejection fraction. While the control subjects rarely show heart rate above 95 bpm, a large fraction of Duchenne patients are above that threshold with normal values of left ventricle ejection fraction but with deteriorated global longitudinal strain.

Figure 3. Posterior distribution of the predicted average for left ventricular ejection fraction (top left), global longitudinal strain (bottom left) and heart rate (bottom right) determined by heart rate-effects. The solid line represents the predicted average and the coloured region the corresponding 95% credible interval for the average. GLS: global longitudinal strain. HR: heart rate. LVEF: left ventricular ejection fraction.
Discussion
As expected, the age effects on the cardiac function outcomes were very different between patient groups: while control subjects did not suffer relevant changes over time, Duchenne patients showed a progressive deterioration. However, the pattern is quite different: while age effects on global longitudinal strain are approximately linear and average group differences can be reliable at younger ages, left ventricle ejection fraction starts to deteriorate at an older age, and group differences can be found close to adolescence. Consequently, we would like to emphasise the fact that strain abnormalities are noted at young ages, supporting the need for early cardiac care.
Cardiac MRI is the gold standard for anatomical, structural, and functional imaging of the heart. Our findings have also been noticed in MRI studies, showing reduced left ventricle ejection fraction and late gadolinium enhancement during the early childhood. Reference Hor, Taylor and Al-Khalidi27,Reference Puchalski, Williams and Askovich28 MRI strain techniques are able to detect myocardial impairment between 7 and 10 years old. Reference Power, O’Grady, Hornung, Jefferies, Gusso and Hofman9,Reference Prakash, Suthar, Sihag, Debi, Kumar and Sankhyan29,Reference Hor, Wansapura and Markham30 MRI is also the best tool to characterise regional wall motion abnormalities. Duchenne-linked cardiomyopathy is non-uniform. It is reported fibrosis and hypokinesia at basal infero-lateral segments at early stages. Reference Magrath, Maforo, Renella, Nelson, Halnon and Ennis31 It could have been studied throughout echocardiography as well, however two-chamber and three-chamber view images should have been included in our study for this purpose.
Regarding our findings of age effects on global longitudinal strain, a linear age effect on global longitudinal strain was also reported by Amedro et al. Reference Amedro, Vincenti and de La Villeon16 with a similar effect size, but confidence intervals for average global longitudinal strain predictions were not shown. Other studies including assessment of age effects on global longitudinal strain Reference Oreto, Vita and Mandraffino15,Reference kui, Xia, Liu, Han, Chen and Li32 performed an age dichotomisation with an arbitrary cut-off (either 8 or 9 years old). In contrast, the age effects in this study were assessed using a continuous function and a linear relationship, having not been assumed before.
Several authors mention that left ventricle ejection fraction and other conventional echocardiographic tools are not sensitive in detecting cardiac dysfunction at an early age. Reference Oreto, Vita and Mandraffino15–Reference Habib and Mohamed21 Our work provides a quantitative model for the age effects on left ventricle ejection fraction, showing a non-linear progression for the average left ventricle ejection fraction and the corresponding uncertainty. In clinical practice, left ventricle ejection fraction is usually dichotomised with a cut-off value around 52–55%. Several studies Reference Oreto, Vita and Mandraffino15,Reference Amedro, Vincenti and de La Villeon16,Reference Taqatqa, Bokowski and Al-Kubaisi19 have reported deterioration of global longitudinal strain with preserved values of left ventricle ejection fraction, i.e. above threshold. Our data also support this behaviour: all our Duchenne patients younger than 15 years had left ventricle ejection fraction above 55% but with a reduced global longitudinal strain. Our recommendation, also held by many others, is to avoid the dichotomisation of quantitative variables, such as left ventricle ejection fraction.
Similarly, several studies reported that the heart rate of Duchenne patients is higher than for the control subjects. Reference Oreto, Vita and Mandraffino15,Reference Amedro, Vincenti and de La Villeon16,Reference Song, Zhang, Wang, Zhang, Sun and Yu18,Reference Taqatqa, Bokowski and Al-Kubaisi19,Reference Habib and Mohamed21,Reference Ryan, Taylor and Mazur33 Increased heart rate at rest should not be overlooked during the cardiological evaluation. It is worthy to note that the heart rate of Duchenne patients was higher than controls even the fact that two Duchenne (12%) patients were under beta-blocker treatment. Two possible mechanisms could be responsible for higher heart rate: dysautonomia in Duchenne patients which could lead to tachycardia Reference Smith, Downey, Williamson and Mizuno34 and/or the haemodynamic compensatory effect to maintain the cardiac output.
All in all, the findings reported in this study are in close agreement with previous studies, but the Bayesian framework provided a probabilistic description of the group differences on the age effects and the heart rate effects on the cardiac outcomes.
Several studies concluded that early treatment with angiotensin-converting enzyme inhibitors and/or beta-blockers in Duchenne patients is associated with a higher overall survival and lower rates of hospitalisation. Reference Viollet, Thrush, Flanigan, Mendell and Allen35,Reference Porcher, Desguerre and Amthor36 However, the assessment of the optimal time to start treatment is a challenge for cardiologists. Reference El-Aloul, Altamirano-Diaz and Zapata-Aldana37 The statistical model proposed in this work can be considered as a valuable tool to assess the relationship between age, heart rate and cardiac outcomes, which provide insight for decision-making about treatment, especially at an early age.
Conclusions
Progressive left ventricular dysfunction in Duchenne patients is one of the key issues and starts at an early age with subtle symptoms. This cross-sectional study provides supporting evidence that global longitudinal strain is an earlier marker of disease progression than ejection fraction in Duchenne patients. Group differences on global longitudinal strain were observed as early as the age of 7, while left ventricle ejection fraction differences were only above 13 years, though being in the normal range.
Study limitations
Several limitations of the study deserve some comments. This was a single-centre and cross-sectional study, but we are aware that longitudinal studies can provide more powerful data to characterise the temporal evolution of muscular dystrophinopathies. This will be the topic of future work. Even though the sample size was relatively small, which is of course a limitation, the Bayesian framework used here allowed us to quantify the uncertainty in model parameters and predictions. It is worthy to mention that the model allows us to quantify not only the credibility of age effects but also its effect size. Therefore, finding such differences between groups means how large the size effect is. We would like to emphasise that the inference is performed in terms of probabilities of the posterior distribution, rather than p-values in a frequentist framework, Reference Althouse, Below and Claggett25 which can be considered as a strength of this study.
In this study, we only analysed the longitudinal strain assessed from the apical four-chamber view. Obviously, a joint analysis including also two- and three-chamber views would allow a more complete assessment of the LV deformation. Similarly, a regional description of the longitudinal strain in segments can provide a more detailed anatomical description. Reference Oreto, Vita and Mandraffino15,Reference Amedro, Vincenti and de La Villeon16,Reference Taqatqa, Bokowski and Al-Kubaisi19
Acknowledgements
None.
Financial support
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. M. Clavero-Adell was supported by a Río Hortega Grant from “Instituto de Salud Carlos III” (CM20/00171).
Competing interest
None.
Ethical standards
The study was conducted in compliance with the Good Clinical Practices protocol and Declaration of Helsinki principles. Approval was granted by Clinical Research Ethics Committee of Aragon (5th February, 2020; number PI20/012).
Consent to participate: Informed consent was obtained from all individual participants older than 16 years old included in the study. Informed consent was obtained from the parents of participants younger than 16.







