Developmental care for hospitalizsed infants is a family-centered care approach that supports individualised care via observation and interpretation of the infant’s behaviour and modification of the environment and caregiving to meet the needs of infants and their families. Developmental care is widely recognised as best practice for medically fragile infants born prematurely or who require surgical intervention after birth;Reference Milette, Martel, Ribeiro da Silva and Coughlin McNeil1,Reference Kenner and McGrath2 however, implementation of developmental care practices occurs mainly in neonatal iICUs and less often for sub-specialty paediatric cardiac ICUs. Over the past decade, literature has emerged calling for developmental care to be integrated into the care of infants with CHD.Reference Torowicz, Lisanti, Rim and Medoff-Cooper3–Reference Butler, Huyler, Kaza and Rachwal7 The Cardiac Neurodevelopmental Outcomes Collaborative recently identified a priority for the CHD field to adapt and implement developmental care interventions in the CHD population.Reference Cassidy, Butler and Briend8 However, wide variability in the clinical practice of developmental care has been consistently demonstrated.Reference Sood, Berends and Butcher9–Reference LaRonde, Connor, Cerrato, Chiloyan and Lisanti12 Barriers identified for paediatric cardiovascular healthcare providers include lack of education, resources, and competing priorities to support developmental care with this specific infant population.Reference Sood, Berends and Butcher9,Reference Miller, Lisanti and Witte10 Given the well-documented risks for neurodevelopmental abnormalities associated with CHD,Reference Marino, Lipkin and Newburger13 and the similarities in brain maturity and structure between infants with CHD and infants born prematurely,Reference Licht, Shera and Clancy14,Reference Wernovsky and Licht15 developmental care is a critical aspect to clinical practice during infant hospitalisation for cardiac surgery.Reference Lisanti, Vittner, Medoff-Cooper, Fogel, Wernovsky and Butler6
One method to reduce variability in practice is a clinical pathway, which is a structured plan of care utilised by the interdisciplinary team to support clinical decision making with detailed steps and evidence-based guidelines.Reference Rotter, Kinsman and James16 Clinical pathways support standardisation in care, with improved patient outcomes and reduced complications.Reference Rotter, Kinsman and James16–Reference Trimarchi, Caruso, Magon, Odone and Arrigoni18 The Cardiac Newborn Neuroprotective Network, a Special Interest Group of the Cardiac Neurodevelopmental Outcome Collaborative, formed a working group of experienced paediatric cardiovascular clinicians and parents to create an evidence-based developmental care pathway to guide clinical practice in hospital settings caring for infants with CHD. Members represented the following paediatric sub-specialties: nursing, psychology, cardiology, critical care, physical therapy, occupational therapy, speech language pathology, nutrition, and parents. Members were assigned specific sections of the pathway based on their area of expertise and completed comprehensive reviews of the literature for each section. This paper presents the clinical pathway content generated by the working group based on evidence in the literature and/or expert consensus where evidence does not exist in the extant literature. The pathway was developed based on underlying assumptions and definitions (Table 1). The “Developmental Care Pathway for Hospitalized Infants with Congenital Heart Disease” (Fig 1) contains three sections:
-
I. Initiate and Document Developmental and Psychosocial Screening on Admission
-
II. Initiate and Document Daily Evidence-based Bundle of Care
-
III. Continue Bundle of Care Daily Until Discharge. Repeat Formal Developmental and Psychosocial Screening Prior to Discharge.
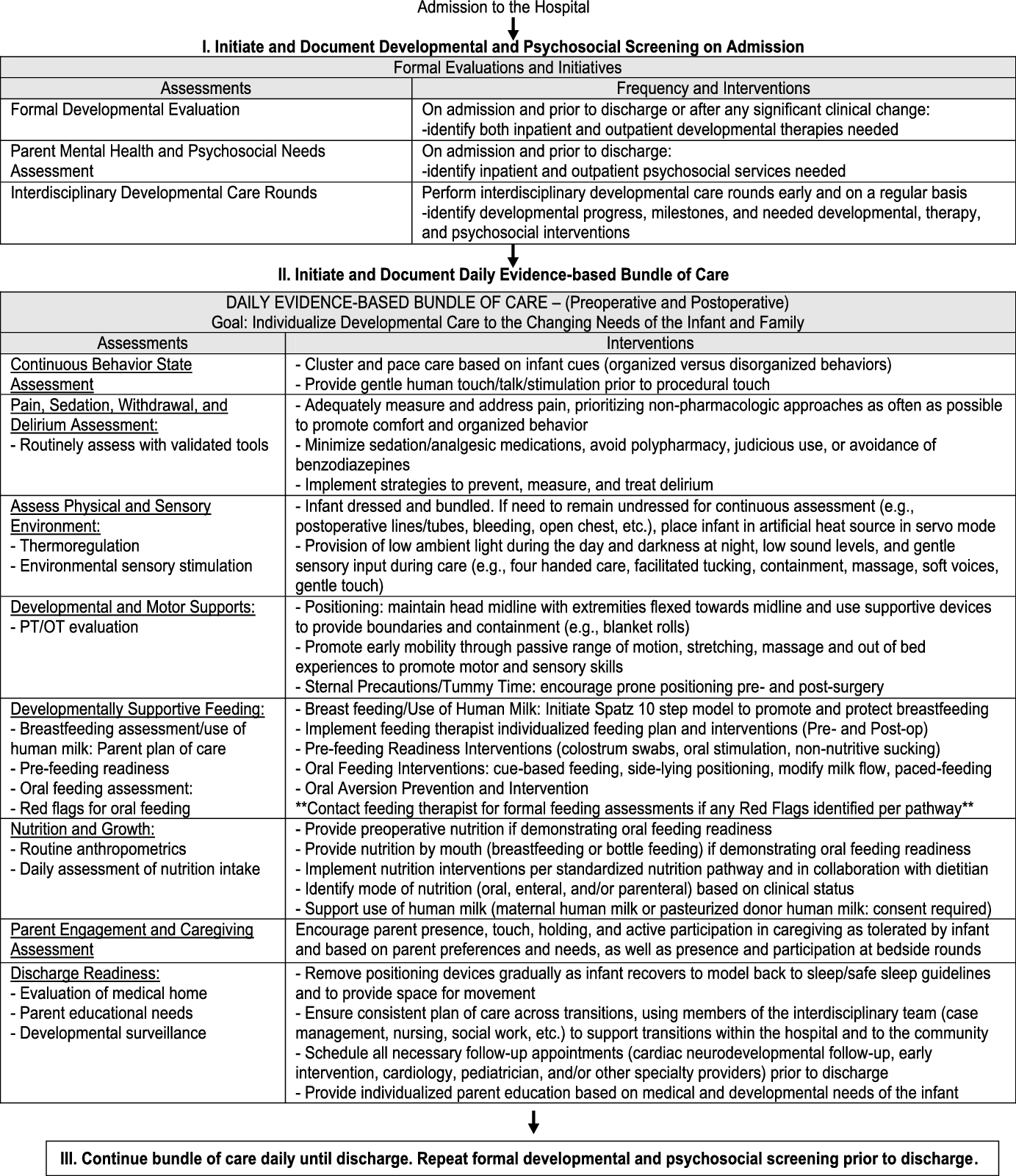
Figure 1. Developmental care pathway.
Table 1. Key assumptions and definitions of the developmental care pathway for hospitalized infants with CHD

PT: Physical therapist, OT: Occupational therapist, SLP: speech language pathologist, ICU: intensive care unit.
We describe each section in detail below and more granular information is available in the tables.
I. Initiate and document developmental and psychosocial screening on admission
Upon admission to the hospital, we recommend formal developmental and psychosocial screening for infants and their parents including documentation of findings in the medical record.
Formal developmental evaluation
Observable infant behaviour is an expression of neurobehavioural status and brain function that can be used to identify infants at risk for developmental delays.Reference Spittle and Treyvaud19 Neurobehavioural assessments are used to determine infants’ developmental strengths and vulnerabilities as well as infants’ ability to attend and engage with environmental stimuli.Reference Butler, Sadhwani and Stopp20 Neurobehavioural assessments may use observation or a facilitated examination to identify patterns of behaviour of infants. An important consideration is to be mindful of the infant’s level of stress during the interaction, as well as understanding what strategies the infant uses to actively participate.Reference Browne, Jaeger and Kenner21 Most infant neurobehavioural assessments require additional training and certification prior to use in clinical settings with infants and families.Reference Lisanti, Vittner, Medoff-Cooper, Fogel, Wernovsky and Butler6,Reference Als22 To fully assess infant state, motor skills, autonomic stability, self-regulation, and social interaction, we recommend developmental assessment measures that are based on the Brazelton Neonatal Behavioral Assessment Scale.Reference Brazelton and Nugent23 Other developmental assessment measures are listed in Table 2.
Table 2. Suggested instruments and clinical tools.

Parent mental health and psychosocial needs assessment
Parents of infants with CHD are at increased risk for mental health challenges such as traumatic stress, anxiety, and depression,Reference Woolf-King, Anger, Arnold, Weiss and Teitel24,Reference Uzark and Jones25 and those who lack social support and resources are more likely to endorse higher stress levels.Reference Visconti, Saudino, Rappaport, Newburger and Bellinger26 Following birth, parents of infants with CHD are exposed to multiple stressors including the ICU environment, cardiac disease diagnosis, newborn cardiac surgery, financial and vocational stress, hospitalisations and critical illness, increased caregiving demands, and disruption to the family system.Reference Golfenshtein, Srulovici and Medoff-Cooper27–Reference Logan, Sahrmann, Gu and Hartman29
We recommend routine parental mental health and psychosocial screening to identify psychosocial services to benefit families during infant hospitalisation and post-discharge.Reference Lisanti30 Examples of parent mental health screening tools are listed in Table 2. Psychosocial assessments include screening for insurance needs, transportation insecurity, food insecurity, housing status, childcare for siblings, employment status, educational concerns, family support, supportive coping strategies, attachment and bonding, understanding of infant clinical status, and potential barriers to adjustment. Psychosocial team members can be utilised for support and resource identification, caregiver support groups, and continued screening and support following hospital discharge.Reference Lumsden, Smith and Wittkowski31
Interdisciplinary developmental care rounds
Many organisations and hospital systems utilise interdisciplinary developmental care rounds as best practice to foster education and collaboration with families and healthcare professionals in providing developmentally appropriate care (Supplementary Table 1).Reference Peterson4,Reference Butler, Huyler, Kaza and Rachwal7,Reference Sood, Berends and Butcher9,Reference Lisanti, Cribben, Connock, Lessen and Medoff-Cooper32 When establishing and performing interdisciplinary developmental care rounds, we recommend identifying consistent team members and timing for rounds to facilitate family presence and participation.Reference Butler, Huyler, Kaza and Rachwal7,Reference Sood, Berends and Butcher9,Reference Griffiths, James-Nunez and Spence33 We recommend initiating interdisciplinary developmental care rounds on admission to establish a baseline and continuing on a regular basis during infant hospitalisation for ongoing care. Documentation and dissemination of recommendations to the bedside care team and family should be provided.
II. Initiate and document daily evidence-based bundle of care
We recommend a daily bundle of care to support individualised developmental care that responds to the changing strengths and areas of concern of the infant and family throughout the hospitalisation. A bundle of care is a group of structured evidence-based interventions that are provided together by the multidisciplinary team to improve patient outcomes. This bundle of care to support individualised developmental care includes both assessment of the infant and family across multiple domains and recommendations for tailored interventions based on the assessments. We review each domain below.
Continuous behaviour state assessment
Assessment
Infant behaviour should be continuously observed (sometimes called cue-based care) to guide the infant’s plan of care and to determine infant strengths and vulnerabilities. Infant behaviour is composed of five subsystems of functioning: autonomic, motor, state, attention/interaction, and self-regulation (Supplementary Table 2). These sub-systems exist simultaneously and mutually influence each other, occurring in continuous interaction with the environment based on the Synactive Theory of Development.Reference Als34 Infant functioning is described and understood in terms of organisation or competencies and disorganisation or vulnerabilities.Reference Als34
Interventions
Consider opportunities to create a calm, nurturing, and welcoming environment.Reference Lisanti, Vittner, Medoff-Cooper, Fogel, Wernovsky and Butler6 Optimise opportunities to cluster and pace caregiving tasks based on infant behaviour to enhance the infant’s emotional development of building trust and security.Reference Browne, Jaeger and Kenner21 Provide the infant with opportunities to respond, pausing after initial greeting, and facilitate behavioural responses that support the infant to self-soothe (e.g., bring their hands to mouth).Reference Browne, Jaeger and Kenner21
Pain, withdrawal, sedation, and delirium assessment
Assessment
Infants with CHD face a variety of painful experiences during their hospitalisations. These unpleasant or unexpected sensory and emotional experiences during early development may lead to permanent alterations in brain processing and development.Reference Anand and Carr35–Reference Maxwell, Fraga and Malavolta38 Optimal pain management requires a multimodal approach. We recommend the use of objective pain assessment tools (Table 2) to avoid untreated pain or excessive analgesia.Reference Hall and Anand37 Typically, acute postoperative pain is greatest in the first 48 hours with a progressive decline over the course of 7 days.Reference Brasher, Gafsous and Dugue39
The assessment and treatment of pain in children can reduce the risk and severity of delirium, a nonspecific disturbance of cognition and consciousness resulting from critical illness and ICU exposure.Reference Smith, Brink, Fuchs, Ely and Pandharipande40 Delirium affects 49% patients in paediatric cardiac ICUs, with 64% of those less than 1 year of age.Reference Smith, Brink, Fuchs, Ely and Pandharipande40 Delirium predisposes patients to long-term complications including decreased verbal and spatial memory and sustained attention, and impaired executive function.Reference Smith, Brink, Fuchs, Ely and Pandharipande40–Reference Staveski, Pickler and Lin42 Delirium is well-studied in paediatric populations, and validated screening instruments exist (Table 2) to enable early recognition.
Interventions
Ultimately, the goal is to prevent, reduce, or limit acute pain in the perioperative period by utilising a combination of pharmacologic and non-pharmacologic interventions.Reference Patel, Trujillo-Rivera and Faruqe43 Sedative medications are a mainstay therapy in the ICU to help patients tolerate the environment and medical/nursing interventions and to reduce anxiety. Sedation should be tailored to the patients’ individual needs utilising goal-directed therapy.Reference Zuppa and Curley44 Sedation levels and assessment for withdrawal should be examined using validated instruments (Table 2). Consideration should be paid to the total dosage and duration of sedation and analgesia medications due to the increased risk of morbidities (withdrawal, altered sleep, delirium, immobility, and potential neurotoxicity).Reference Patel, Trujillo-Rivera and Faruqe43 Specifically, judicious use or avoidance of benzodiazepines may reduce the likelihood of developing delirium.Reference Smith, Besunder and Betters45,Reference Feroz and Donnelly46
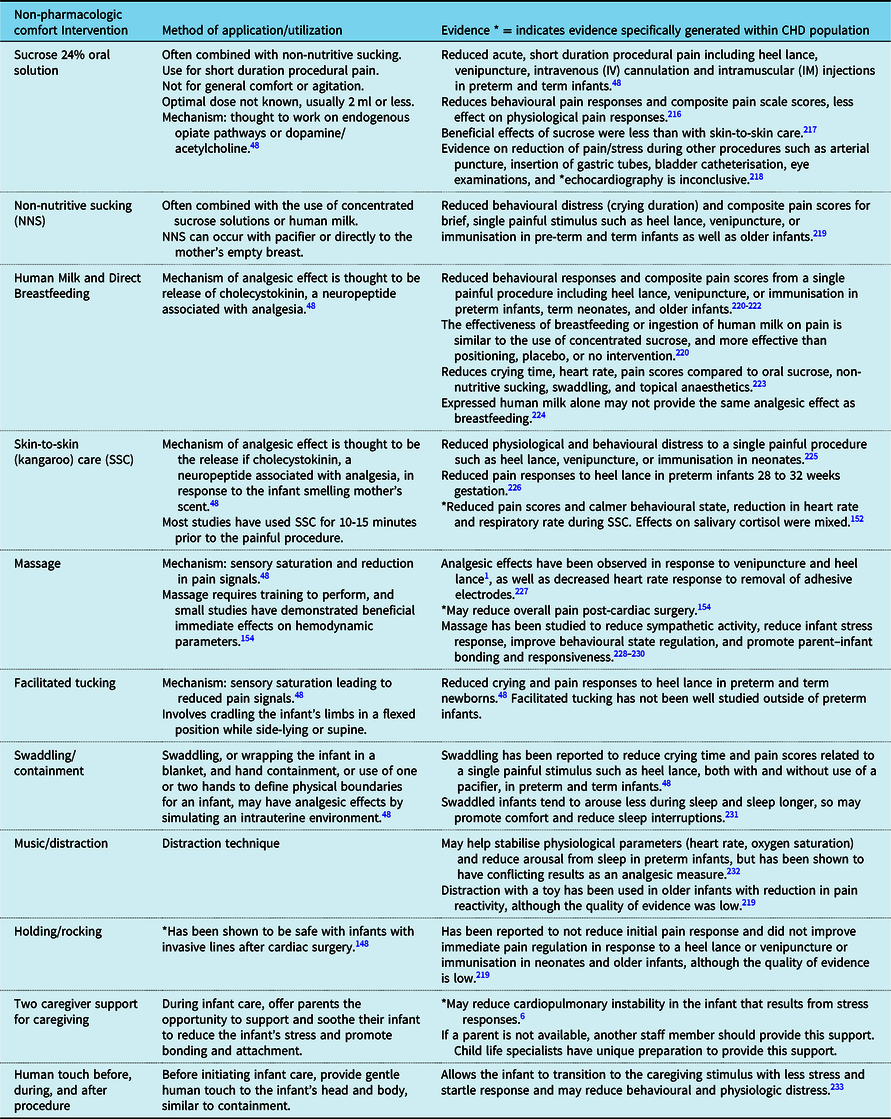
We recommend the incorporation of non-pharmacologic comfort measures into the care of CHD infants to prevent and manage pain and agitation in acute postoperative setting or with treatment of withdrawal and delirium (Table 3).Reference Smith, Besunder and Betters45 Non-pharmacologic comfort measures include a broad range of interventions, with varying degrees of evidence for each.Reference McNair, Campbell-Yeo, Johnston and Taddio47,Reference Mangat, Oei, Chen, Quah-Smith and Schmolzer48 Our recommendation is to include as many as possible, based on the individualised infant response to the intervention and parental preferences.Reference McNair, Campbell-Yeo, Johnston and Taddio47 Encouraging parents to provide non-pharmacologic measures increases parental engagement to provide comfort to their child.Reference McNair, Campbell-Yeo, Johnston and Taddio47 Non-pharmacologic comfort measures should not replace adequate analgesia, but should be used in combination,Reference McNair, Campbell-Yeo, Johnston and Taddio47 and may also be effective to treat withdrawal and delirium symptoms by normalising the environment.Reference Feroz and Donnelly46
Table 3. Non-pharmacologic comfort interventions.
Assess physical and sensory environment: thermoregulation
Assessment
Although minimal research has studied thermoregulation in infants with CHD, the National Association of Neonatal Nurses developed general guidelines for temperature assessment in medically fragile newborns and infants (Table 2).49 Infants with CHD, whether born premature or full-term, require close monitoring of temperature during hospitalisation due to risks of heat loss in the ICU / intraoperative environment and the associated interventions of critical illness.
Interventions
Infants receiving artificial heat sources in the pre or postoperative period should receive continuous temperature assessment while in use.49,Reference Knobel and Holditch-Davis50 Newly born infants with CHD who are >34 weeks’ gestation and not at risk for increased oxygen consumption should be admitted onto a pre-warmed radiant warmer/bed surface in servo control mode.49,Reference Kusari, Han and Virgen51 If there are concerns about the artificial heat reaching the infant because of sterile drapes or other procedures like echocardiograms, consider a thermal warming pad. Thermal warming pads may provide heat for up to two hours but may need to be replaced sooner depending on manufacturer guidelines. Place a single layer blanket in between the infant and the thermal warming pad. Incubators are helpful when infants with CHD are unable to maintain a body temperature of 36.5 °C axillary, unable to demonstrate adequate weight gain, are <34 weeks’ gestation or <1500 g, or are from an outside hospital and previously maintained in an incubator.49,Reference Altimier52 Consider an incubator for infants ≥34 weeks’ gestation who are demonstrating signs of increased oxygen consumption.49,Reference Flenady and Woodgate53
Consider use of the following during patient transport: pre-warmed artificial heat source, power supply attached to artificial heat source, warm blankets, hat, pre-warming infant prior to transport (goal temperature 36.5 °C), and thermal warming pad.49,Reference Brozanski, Piazza and Chuo54–Reference Harer, Vergales, Cady, Early, Chisholm and Swanson57 When transitioning out of any artificial heat source into an open crib, infants should be dressed and bundled with a blanket(s) and demonstrate normothermia and weight gain for 2–3 days before discharge.49,Reference Shankaran, Bell and Laptook58 While maintaining continuous temperature monitoring, manually assess axillary temperature frequently until the infant’s temperature is stable.49,Reference Shankaran, Bell and Laptook58 Evidence varies related to device mode (air versus servo control) during weaning.49
Assess physical and sensory environment: environmental sensory stimulation
Assessment
Infant behaviour should be continuously observed (Supplementary Table 2) to determine the infant’s sensory experience and its influence on the infant and to provide opportunities for environmental modification. Interpretation of infant responses to sensory stimulation allows for direct attention to and response to demands from the physical and sensory environment that influence infant behavioural patterns.Reference Butler, Huyler, Kaza and Rachwal7,Reference Als34 Thus, all actions and reactions are dependent on the ability to interpret and respond to sensory information to support the infant’s self-regulation and thresholds of coping.Reference Browne, Jaeger and Kenner21
Interventions
Sensory interventions should aim to regulate the exposure to environmental stimuli, but not devoid the environment of all stimuli, with a goal of creating a home-like environment. We recommend the provision of low ambient light during the day and darkness at night, low sound levels, and gentle sensory input during care, including: four-handed care (involving two individuals in caregiving where one completes the care and one supports the infant), facilitated tucking, containment, massage, soft voices, and gentle touch. To support the auditory system, we support the recommendations of the American Academy of Pediatrics committee on environmental health:59
-
1. Incorporating a system of regular sound assessment in the ICU and within incubators. Ideally, a sound level exceeding 45 dB is avoided.
-
2. ICUs should develop and maintain a programme of sound reduction and management. This can include decreasing the sound levels in the entire unit, decreasing sound within the infant’s bedside, reducing loud alarms and phone ringers, lowering voices to a soft whisper, reducing the sounds of machinery, running water, and closing lids/doors.Reference Graven60
-
3. Care practices should provide opportunities for the infant to hear parent voices live during parent–infant interaction, including book reading.Reference Neri, De Pascalis and Agostini61,Reference Erdei, Klass and Inder62 Earphones and other devices attached to the infant’s ears for sound transmission should always be avoided.Reference Graven60,Reference Morag and Ohlsson63
To support the visual system, remember that the ideal focal distance for term infants is 10–12 inches and gradually increases with time.Reference Graven and Browne64 A parent’s face is the most important visual input. Color perception does not fully develop until 2–3 months of age. Closely monitor infant reactions to determine if they are regulating appropriately and tolerating stimuli. High color-contrast toys and mobiles should only be used for short periods of time (approximately 10–15 minutes) based on infant cues for tolerance. Bright lights disrupt infant rest and sleep.Reference Kenner and McGrath2 We recommend providing cycled lighting, which is low ambient light during daylight hours and darkness at night. This improves weight gain and increases sleep time in hospitalised infants.Reference Morag and Ohlsson63 When infants are in incubators, the incubators can be partially covered at night to support the development of the infant’s circadian rhythm while allowing visualisation of the infant.
Smell and taste are powerful sensory inputs that develop prenatally and are important in the transition to postnatal feeding. In addition to the importance in recognition of the mother, smell and taste initiate metabolic pathways that promote digestion and metabolic control.Reference Bloomfield, Alexander, Muelbert and Beker65 Young infants who are hospitalised and especially those on tube feedings should receive the taste and smell of mother’s milk and smell of family while held skin-to-skin during feeding, even tube feedings. Providers should also be mindful of the taste and smell of hand cleaning materials, medications, materials placed on infant lips and face, and perfumes on staff.
The vestibular system begins to develop in the womb. Postnatally, the infant is strengthened and stimulated through movement and changes in position. Throughout infancy and toddlerhood, movements like rocking, swinging, rolling, crawling, walking, and running all provide input for a healthy vestibular system.Reference Pineda, Roussin, Heiny and Smith66 Given the potential for those movements to be limited in hospitalisation, maintaining changes in position and movement are important for continued development of the vestibular system, with interventions such as therapeutic positioning, range of motion, movement of the extremities or body, or therapeutic facilitation of the muscles.Reference Browne, Jaeger and Kenner21 Attention should also be paid to the tactile sensitivity of an infant and particularly passive touch (sensitivity to stimulation imposed on the skin).Reference Browne, Jaeger and Kenner21 The hospitalised infant is exposed to many tactile stimulations and often show higher sensitivity to tactile stimuli.Reference Andre, Durier and Beuchee67 We recommend consideration of all objects touching the infant’s skin and body. Allow for the availability of infant hands, face, and other areas of skin to remain as free from lines, tubing, and tape as possible so they can feel their own body, the skin of their parents, and soft materials/blankets.
Developmental and motor supports
Assessment
Infants with CHD who undergo surgery in the first six months of life are at risk for delayed motor development as well as musculoskeletal impairments.Reference Clifton, Cruz, Patel, Cahalin and Moore68 A developmental evaluation by a physical and/or occupational therapist integrates many aspects of a standardised assessment, including examination of environmental factors, motor function, neuromotor development, sensory processing, range of motion, reflex integrity, social interaction, and family needs.69 A developmental evaluation is specifically tailored to respect the unique needs of a critically ill infant, minimising stress and monitoring physiologic state throughout.Reference Sweeney, Heriza, Blanchard and Dusing70
Interventions
We recommend a physical and/or occupational therapy consult upon admission as well as ongoing evaluation at regular intervals to ensure individualised developmental therapy supports are initiated early in the infant’s hospitalisation.Reference Jara, Jacobs and Reilly71 These team members specialise in movement and posture, providing a unique opportunity to affect the shape of the musculoskeletal system and motor organisation of infants and concurrently supporting parents and caregivers to optimise infant neurodevelopment.Reference Clifton, Cruz, Patel, Cahalin and Moore68
We further recommend intentional therapeutic positioning of infants bundled with routine nursing care that includes head in midline, extremities flexed towards midline, and use of supports to provide boundaries and containment.Reference Jara, Jacobs and Reilly71 Providing boundaries to mimic the resistance in utero such as a swaddle or supports for positioning will allow dynamic activity to occur. This promotes a calm state and allows for improved self-regulation which can optimise cognitive and social-emotional outcomes.Reference Waitzman72 Facilitated positioning throughout the hospitalisation promotes gross motor skills. Without proper positioning, abnormalities may occur in the musculoskeletal system such as retracted shoulders, hyperextension of the head and neck, plagiocephaly, and external rotation of the lower extremities.Reference Jara, Jacobs and Reilly71 Motor abnormalities are also observed such as poor head control, poor reaching skills, and resistance to postural changes.Reference Lisanti, Vittner, Medoff-Cooper, Fogel, Wernovsky and Butler6 Position of infant should be changed every 2 to 3 hours or with bundled nursing care times as appropriate. Positioning an infant in one position for an extended period can contribute to skeletal deformity, muscle shortening, and restricted mobility of joints.Reference Gerard, Harris and Thach73
Consider early mobility which includes range of motion exercises (active, passive, and active assisted), massage, and out of bed experiences (holding, infant seat, swing, etc.).Reference Cameron, Ball and Cepinskas74,Reference Kanejima, Shimogai, Kitamura, Ishihara and Izawa75 Tummy time, or prone positioning, should be encouraged preoperatively and when prone precautions are cleared.Reference Jennings, Sarbaugh and Payne76,Reference Uzark, Smith, Yu, Lowery, Tapley, Romano and Butcher77 Prone positioning has multiple benefits, such as improving ventilation and decreasing work of breathing.Reference Clifton, Cruz, Patel, Cahalin and Moore68 Research supports more timely attainment of gross motor skills for infants placed in prone positioning.Reference Jennings, Sarbaugh and Payne76,Reference Uzark, Smith, Yu, Lowery, Tapley, Romano and Butcher77 Tummy time progression, as well as plagiocephaly and torticollis prevention strategies can be demonstrated with parents even when infant mobility is restricted due to sternal precautions while hospitalised.Reference Waitzman72
Developmentally supportive feeding
Assessment
Breastfeeding assessment/use of human milk. We recommend parents experience the process of informed decision making for the use of human milkReference Nyqvist, Haggkvist and Hansen78 using the Spatz 10 Step Model for Human Milk and Breastfeeding for Vulnerable Infants (Supplementary Table 3).Reference Spatz79 This model has been effectively implemented globally for infants requiring intensive care,Reference Spatz80 and in 2021 was adapted as the national model for human milk and breastfeeding in the United States by the Association of Women’s Health Obstetric and Neonatal Nurses..81 The model demonstrated improved human milk and breastfeeding outcomes exceeding national targets in several high-risk infant surgical groups.Reference Edwards and Spatz82–Reference Froh, Schwarz and Spatz84
Pre-feeding readiness assessment. We recommend all infants with CHD receive a pre-feeding readiness assessment prior to introducing oral feeding during hospitalisation. An infant is ready to be fed orally when demonstrating the following conditions:
-
Awake and alert state: Active engagement for efficient and safe oral feeding is highly dependent on the ability of the infant to achieve and maintain awake state.Reference Thoyre, Pados, Shaker, Fuller and Park85 Oral feeding efficiency improves in alert state compared to sleep state prior to oral feeding in stable preterm infants.Reference Griffith, Rankin and White-Traut86
-
Demonstrating feeding cues with established non-nutritive suck on a pacifier: The infant must achieve and maintain autonomic and state organisation to maintain stability in increasingly complex motor tasks such as non-nutritive sucking. We recommend attention to stress cues during engagement in non-nutritive suck, which may include increased respiratory rate, arching, munching, and pulling off or away from pacifier.Reference Gennattasio, Perri, Baranek and Rohan87,Reference Ross and Philbin88
-
Maintain physiologic stability with attention to level of respiratory support: Infants with tachypnoea may have challenges regulating a coordinated suck/swallow/breathe sequence. Limited evidence exists on feeding safety on non-invasive positive pressure respiratory support, with currently no research exclusive to the full-term cardiac infant.Reference Canning, Clarke, Thorning, Chauhan and Weir89 Without sufficient guidelines for feeding infants on respiratory support, careful consideration of the infant’s clinical picture is needed for smaller bolus oral feeding when on stable respiratory support.Reference Raminick and Desai90
We recommend the Readiness section of the early feeding skills assessment be administered by a feeding therapist to objectively determine if the infant can demonstrate appropriate motor state and feeding cues prior to offering breast or bottle.Reference Thoyre, Pados, Shaker, Fuller and Park85
Oral feeding assessment. We recommended utilising a cue-based feeding assessment, with continuous evaluation of infant behavioural state, infant cues to engage and disengage from feeding, oral motor function for latching, sucking and milk extraction, and regulation of swallowing and breathing.Reference Thoyre, Holditch-Davis, Schwartz, Melendez Roman and Nix91–Reference Shaker93 Further oral feeding assessment includes response to feeding supports, haemodynamic and respiratory stamina and stability with feeding activity, and sustaining endurance for adequate oral intake to maintain growth and development.Reference Griffith, Rankin and White-Traut86 Infants with CHD are at risk for tachypnoea, tachycardia, and decreased oxygen saturations during oral feedings, resulting in difficulties maintaining appropriate and safe suck/swallow/breath coordination during oral feeds, as well as have a higher prevalence of gastroesophageal reflux in comparison to those without CHD.Reference Pereira Kda, Firpo and Gasparin94,Reference Jones, Desai and Fogel95 This necessitates continuous screening and evaluation for swallowing difficulty/aspiration risk and gastroesophageal reflux. Oxygen desaturations, increased work of breathing, and coughing/choking during feeds are overt signs of swallowing difficulties, placing infant at high risk for aspiration.Reference Pereira Kda, Firpo and Gasparin94 Common symptoms of gastroesophageal reflux include discomfort, irritability, excessive crying, back arching, feeding refusal, and/or frequent emesis.Reference Pados and Davitt96
To date, no standardised objective feeding assessments specific to the unique needs of infants with CHD have been validated; however, assessments specific to preterm newborns exist and may have applicability with infants who have CHD.Reference Jones, Desai and Fogel95,Reference Jadcherla, Vijayapal and Leuthner97,Reference Malkar and Jadcherla98 Standardized assessments and algorithms evaluate feeding readiness, oral motor function, swallowing skills, and respiratory regulation to determine the infant’s challenges for appropriate feeding and to guide intervention (Table 2).Reference Thoyre, Pados, Shaker, Fuller and Park85,Reference Ehrmann, Harendt and Church99,Reference Lau and Smith100
“Red Flags” during oral feeding. Red flags are signs to discontinue oral feeding or to consider feeding modifications or additional supports. Continued feeding when red flags are present can result in negative oral feeding experiences and ultimately oral aversion.
-
Behavioural state and subsystem changes (Supplementary Table 2)
-
Clinical signs of dysphagia: milk dribbling from the mouth, coughing during feeding, wet breath sounds, milk residual in oral cavity, fatigue, gulping, multiple swallows, pulling off the nipple, desaturations, cyanosisReference Pereira Kda, Firpo and Gasparin94
-
Signs of feeding intolerance or gastroesophageal reflux: discomfort, irritability, excessive crying, vomiting, back arching, feeding refusal, mouthing, stridorReference Gulati and Jadcherla101
Interventions
Breastfeeding/use of human milk. Follow the Spatz 10 Step Model for human milk and breastfeeding for vulnerable infants (Supplementary Table 3) to support parents in their use of human milk and/or breastfeeding.Reference Spatz79
Pre-feeding readiness interventions. Oral care with colostrum has known benefits in premature infants, including decreased inflammatory response in postsurgical infants, stimulation of the infant’s immune system, decreased risk of infection, and faster attainment to full oral feeding.Reference Gephart and Weller102 We recommend administering fresh colostrum via a saturated sterile cotton swab (0.2 ml total) on the tongue, gums, and inner cheeks and repeating every 3–4 hours if the infant is tolerating well.Reference Gephart and Weller102–Reference Sauer and Altmiller104 Even while intubated, oral care, and stimulation with colostrum/breast milk is beneficial in newborn infantsReference Thibeau and Boudreaux105 with a decreased incidence of thrush and ventilator-associated pulmonary infections in infants after cardiothoracic surgery.Reference Yu, Huang and Xu106
Oral stimulation includes non-nutritive sucking and offering a pacifier dipped in expressed human milk or formula. A combination of oral stimulation may improve readiness for initiation of oral feeding, attainment of total oral nutrition, reduce time to full oral feeding, and reduce length of stay following cardiac surgery.Reference Gennattasio, Perri, Baranek and Rohan87,Reference Yu, Huang and Xu107,Reference Coker-Bolt, Jarrard, Woodard and Merrill108
Cue-based feeding. We recommend use of infant cue-based feeding as the standard oral feeding intervention for infants with CHD. This approach emphasises the quality of the feeding experience over the volume consumed. Cue-based feeding intervention involves observing an infant’s subsystem signs of organisation to initiate and advance oral feeding while understanding stress signs or subsystem disorganisation to alter oral feeding techniques and provide additional support for the infant and parents (Supplementary Table 2). Research demonstrates that cue-based feeding reduces length of stay and improves weight gain in preterm infants.Reference Lubbe92
Individualised feeding plans. We recommend use of individualised feeding plans that are attentive to the infant’s unique developmental and physiologic needs in line with family-centered care. Individualised feeding plans are associated with improved oral feeding outcomesReference Jadcherla, Peng and Moore109,Reference Lubbe, Van der Walt and Klopper110 and should be frequently reevaluated by the feeding team with interdisciplinary collaboration based on the infant and family’s unique needs. Example feeding plan interventions may include:
-
Prioritisation of feeding the infant at the breast if that is the parent’s goal.
-
Infants should be prepared for direct breastfeeding through skin-to-skin care and non-nutritive experience at the breast.
-
Modification of the rate of milk flow from a bottle nipple, which may improve physiologic stability during bottle feeding in term and preterm infants.Reference Daley and Kennedy111,Reference Pados, Park and Dodrill112
-
Side-lying positioning stabilises oxygen saturation during oral feeding, improve chest wall movement by decreasing the effects of gravity on rib cage expansion. This position may be beneficial for slowing bolus flow by decreasing hydrostatic pressure in the bottle compared to upright positioning.Reference Park, Thoyre, Knafl, Hodges and Nix113
-
Co-regulation/paced feeding provides regular pauses or breaks for breathing during an oral feed to allow for improved minute ventilation and decreased respiratory effort.Reference Thoyre, Holditch-Davis, Schwartz, Melendez Roman and Nix91
Oral aversion prevention and intervention. Prevention of oral feeding aversion and compromised feeding is imperative for successful oral feeding and influenced by care practices during hospitalisation for infants with CHD.Reference Goldstein, Watkins and Lowery114 To reduce risk of prolonged feeding challenges, both early exposure to feeding opportunities and early identification of infant vulnerabilities during feeding, gastrointestinal issues and swallowing disorders is imperative.Reference Gulati, Sultana and Jadcherla115,Reference Pados, Hill, Yamasaki, Litt and Lee116 Interventions that support prevention of oral aversion include:
-
Early identification of infant stress during feeding: Stressful feeding experiences can result in establishment of altered neural pathways especially during the initial stages of oral feeding trials which can lead to negative consequences on feeding skill development and desire to feed orally.Reference Shaker93
-
Early identification of gastrointestinal issues: Infants with CHD are at increased risk of feeding and swallowing disorders and gastrointestinal complications.Reference Jadcherla, Vijayapal and Leuthner97,Reference Malkar and Jadcherla98,Reference Rosen, Vandenplas and Singendonk117 Diagnostic assessments in collaboration with a paediatric gastroenterologist can be beneficial to assess potential underlying mechanisms which may contribute to feeding challenges.Reference Rosen, Vandenplas and Singendonk117
-
Early management of swallowing disorders: Infants with CHD are at high risk for swallowing difficulties and should be clinically evaluated by a healthcare professional specialising in dysphagia using diagnostic studies (Table 2).
Nutrition and growth
Infants with CHD are known to be at risk for poor growth,Reference Anderson, Beekman and Eghtesady118–Reference Williams, Zak and Ravishankar122 which negatively influences neurodevelopment.Reference Medoff-Cooper, Irving and Hanlon123,Reference Ravishankar, Zak and Williams124 Infants with CHD may experience growth failure secondary to a variety of factors including suboptimal energy intake, oral aversion, early satiety, increased metabolic demands, and gastrointestinal disturbances impairing motility and/or absorption.
Assessment
Close assessment of growth is imperative to monitor the adequacy of nutrition intake. Routine anthropometric assessments should include daily measures of weight and at least weekly measurement of head circumference and length and consideration of weight-for-length metrics.Reference Green Corkins and Teague125–Reference Goldberg, Becker and Brigham127 Anthropometrics are vital to assist in weight-adjusting nutrition provisions to maintain adequate growth velocity and to identify early concerns for growth failure. Daily intake of calories, proteins, lipids, and macro- and micro-nutrients should be closely monitored and adjusted based on individualised needs.Reference Schwalbe-Terilli, Hartman and Nagle128–Reference Wong, Ong, Han and Lee131 Registered dietitians are essential members of the interdisciplinary team who collaborate to individualise recommendations to optimise nutrition support, monitor growth of infants during hospitalisations, and provide support for feeding intolerance.Reference Gentles, Mara and Diamantidi132,Reference Lisanti, Savoca and Gaynor133
Interventions
We recommend the use of clinical nutrition pathways as a standardised approach to nutrition considering the positive outcomes demonstrated in infants with CHD.Reference Wong, Ong, Han and Lee131,Reference Gentles, Mara and Diamantidi132,Reference Brown, Forbes, Vitale, Tirodker and Zeller134,Reference Hamilton, McAleer and Ariagno135 Specifically, clinical nutrition pathways for infants with CHD have (1) shortened the time to achieve goal calories,Reference Braudis, Curley and Beaupre136,Reference Furlong-Dillard, Neary and Marietta137 (2) increased the percent of patients meeting their calorie and protein goals,Reference Kaufman, Vichayavilas and Rannie138 (3) decreased the incidence of necrotising enterocolitis,Reference del Castillo, McCulley and Khemani139 (4) decreased the duration of postoperative parenteral nutrition,Reference Braudis, Curley and Beaupre136,Reference Furlong-Dillard, Neary and Marietta137 (4) decreased hospital length of stay,Reference Simsic, Carpenito and Kirchner129,Reference del Castillo, McCulley and Khemani139 and (5) improved weight-for-age Z-score change over hospital length of stay.Reference Ehrmann, Harendt and Church99,Reference Lisanti, Savoca and Gaynor133,Reference Kaufman, Vichayavilas and Rannie138 Despite variation in content across published pathways,Reference Slicker, Hehir and Horsley140 standardised nutrition pathways minimise variability of nutrition care between providers within institutions.
Institutional pathways for both implementing feeding and nutrition are helpful. Key components in a pathway should include the safety of preoperative enteral feeding, use of parenteral and enteral nutrition in the postoperative period, frequent anthropometric measurements, avoidance of ad lib oral feeding without clearly defined volume and caloric goals, and consultation with a registered dietitian. Following cardiac surgery, infants often require parenteral and enteral nutrition until clinical status and postoperative dysphagia improves and the infant is demonstrating pre-feeding readiness.Reference Lisanti, Savoca and Gaynor133,Reference Slicker, Hehir and Horsley140 While infant cue-based feeding is an integral component to developmental care, infants in the postoperative period that are ad lib feeding should ideally have defined volume and calorie goals to prevent suboptimal nutrition intake. Infants experiencing difficulties meeting nutrition goals should be supplemented with enteral tube feedings to prevent undernutrition. Enteral nutrition may also be indicated in infants with vocal cord dysfunction or gastrointestinal difficulties.Reference Lisanti, Savoca and Gaynor133,Reference Marino, Johnson and Davies141,Reference Savoca, Horsley, Konek and Becker142
Parent engagement/caregiving
Assessment
An underlying assumption of this pathway is that parents are the primary caregivers for their infants (Table 1). Unfortunately, infants are often separated from their parents, which contributes to parental stress arising from parental role alteration.Reference Lisanti, Golfenshtein and Medoff-Cooper28 Parental role alteration is a perceived stressor that has repeatedly been found in the cardiac ICU setting to uniquely contribute to both anxiety and depressive symptoms in parents.Reference Lisanti, Allen, Kelly and Medoff-Cooper143–Reference Lisanti, Kumar and Quinn145 We recommend that the interdisciplinary team regularly review parent participation at the bedside to determine how to best support families in the care of their child.
Interventions
We recommend that parents be incorporated into all aspects of care, including but not limited to decision making for the plan of care, medical and developmental rounds, emergent and non-emergent medical procedures, comfort measures, and routine infant care such as feeding, diaper changing, dressing, positioning, and holding.Reference Lisanti, Allen, Kelly and Medoff-Cooper143–Reference Lisanti, Kumar and Quinn145 We also recommend removing any organisational barriers for parent presence. Parents should be encouraged and supported to provide direct care to their infants during hospitalisation, including any of the non-pharmacologic comfort measures described in this pathway (Table 3). We recommend supporting parent–infant interactions to promote comfort and reduce stress for infants and their parents. One specific measure that can be uniquely provided by parents is skin-to-skin care, or kangaroo care, which occurs when an infant dressed only in a diaper is held on a parent’s bare chest. Parents participating in skin-to-skin care report positive social-emotional responses, decreased perceived stress, increased parent–infant attachment, decreased post-partum depression, and increase breast milk production.Reference Lisanti, Demianczyk and Costarino146,Reference Ludington-Hoe147 Evidence-based standards and procedures for holding and skin-to-skin care are carefully reported and described.Reference Lisanti, Helman and Sorbello148,Reference Lisanti, Buoni and Steigerwalt149 Research demonstrates that skin-to-skin care in infants with CHD in the pre- and post-operative periods is safe and beneficial.Reference Harrison and Brown150–Reference Lisanti, Demianczyk and Costarino152 We recommend that centres caring for infants with CHD develop evidence-based holding guidelines and procedures for safe transfer and parental holding opportunities to promote parental bonding and holistic care.Reference Harrison5 Reevaluation of holding or skin-to-skin care readiness is recommended to occur daily on rounds. If the infant is very ill and should remain in the bed, parents and caregiving staff are recommended to provide positive sustained touch.Reference Harrison5,Reference Harrison, Williams, Berbaum, Stem and Leeper153,Reference Harrison, Brown and Duffey154
Discharge readiness
Assessment
Comprehensive discharge planning for infants with CHD includes individualised content regarding the developmental and psychosocial needs of infants and their families. Discharge readiness assessment includes ongoing evaluation of the medical home during the inpatient hospitalisation and assessment of parent educational and resource needs.155 This includes daily evaluation of the family (social support, resources, and coping strategies) during the ICU stay and as the infant is de-intensified. Parents may have educational or language barriers, experience emotional struggles, and complex family systems impacting their processing of discharge information and safe transition home. Parents require comprehensive information prior to discharge including, but not limited to, infant medical care (such as infant cardiopulmonary resuscitation, placement and management of supplemental tube for medications and feedings, home equipment orientation, and other medical needs such as oxygen, and special formula instructions), infant safety and caregiving (safe sleep, bathing, monitoring weight, and feeding), and supports for neurodevelopment including referrals to early intervention services, anticipatory guidance on the infant’s developmental opportunities at home, and connection with outpatient developmental services.Reference Mannarino, Michelson, Jackson, Paquette and McBride156
Interventions
We recommend incorporating the developmental and psychosocial needs of the infant and family into the discharge planning process. An integral part of the discharge readiness plan is ensuring a consistent and smooth transition plan of care across service lines (e.g., cardiac ICU to acute care unit to home), utilising available resources and key interdisciplinary team members. As the infant recovers, providers can gradually remove positioning devices to model safe sleep guidelines. Parents or other family caregivers should be provided a comfortable space to room-in with their infant prior to discharge.Reference Jaafar, Ho and Lee157 Rooming-in would ideally be offered as early and as often as possible to provide parents ongoing contact with their child, opportunities to increase bonding with their child, as well as learn, practice, and become confident in the skills required to care for a medically complex infant after discharge.
All follow-up appointments including developmental follow-up and early intervention should be scheduled prior to discharge. Discharge planning should include arranging timely follow-up appointments with primary care, cardiology and other specialist teams, therapists, nutrition, and social work, as needed.Reference Hartman and Medoff-Cooper158 Comprehensive neurodevelopmental follow-up is critical to the ongoing evaluation following discharge of infants with CHD and can include cardiac, post-intensive care, or other neurodevelopmental follow-up team.Reference Marino, Lipkin and Newburger13 For all infants with a supplemental feeding tube, we recommend close follow-up by a registered dietitian and feeding therapist to closely monitor growth and weight-adjust feeding regimens. Research has demonstrated that despite referral by practitioners for early intervention, only about half of high-risk infants with CHD receive early intervention services post-discharge at the time of outpatient neurodevelopmental evaluation and many never received early intervention.Reference Lipkin and Macias159 Discharge education and paperwork should clearly outline the developmental care plan, referral to early intervention, and contact information for the neurodevelopmental follow-up team.
III. Continue bundle of care daily until discharge
Repeat formal developmental and psychosocial screening prior to discharge
As described in previous sections, the components of the developmental care bundle should be continued throughout the infant’s hospitalisation and individualised based on the changing needs of the infant and family. We further recommend repeating a developmental assessment of the infant as well as psychosocial screening of parents (Table 2) prior to discharge to ensure that the family is connected with the appropriate resources post-hospitalisation.
Discussion and conclusions
Neurodevelopmental concerns are highly prevalent in infants with CHD.Reference Marino, Lipkin and Newburger13 After thoroughly reviewing current literature and forming expert and stakeholder consensus, the Cardiac Newborn Neuroprotective Network, a Special Interest Group of the Cardiac Neurodevelopmental Outcome Collaborative, generated an evidence-based developmental care pathway for hospitalised infants with CHD. The pathway includes standardised developmental assessment and parent mental health screening, along with the implementation of a daily developmental care bundle, which incorporates individualised assessments and interventions to tailor care to the needs of infants with CHD and their families. Hospitals caring for infants with CHD are encouraged to adopt this developmental care pathway and track predetermined metrics and outcomes using a quality improvement framework. Many metrics could be followed based on the assessments and instruments outlined in Table 2. We encourage any hospital, centre, or inpatient unit adopting this pathway to closely examine whether documentation fields are available for all developmental care practices and ensure that fields are created where needed in the electronic health record.Reference Miller, Elhoff and Alexander11 Future work is recommended to generate both short- and long-term neurodevelopmental and psychosocial outcomes of this pathway that would greatly add to the body of literature on developmental care for infants with CHD. We acknowledge that where research was lacking in CHD, we applied literature from other populations such as prematurely born or critically ill infants. Continued evaluation of developmentally supportive care interventions through implementation and dissemination of high-quality research and quality improvement investigations are crucial to develop evidence to support best practices in this vulnerable population of infants and their families.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1047951123000525
Acknowledgements
The members of the Cardiac Newborn Neuroprotective Network would like to thank the Publications Committee of the Cardiac Neurodevelopmental Outcome Collaborative for their thoughtful review of this manuscript.
Financial support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Conflicts of interest
None.







