Techniques of ductal stenting for infants with ductal-dependent pulmonary blood flow have evolved quickly over the past one to two decades. Reference Aggarwal, Petit, Glatz, Goldstein and Qureshi1,Reference Promphan and Qureshi2 Ductal stent has been used successfully in infants with pulmonary atresia and intact ventricular septum following valve perforation. Reference Aggarwal, Petit, Glatz, Goldstein and Qureshi1,Reference Brown, Cools, Boshoff, Heying, Eyskens and Gewillig3 Ductal stent flow reduction may need to be considered, especially in small infants, to avoid the risk of postprocedural decompensated congestive heart failure. Reference Promphan and Qureshi2,Reference Demir, Cools and Gewillig4 In this report, we presented a novel technique to restrict ductal flow in a premature neonate.
A twin male neonate was born at 36 weeks’ gestation with a birth weight of 2.5 kg. He was prenatally diagnosed with pulmonary atresia and intact ventricular septum. Due to prostaglandin shortage, we recommended trans-catheter interventions on the first day of life. A cardiac catheterisation was performed via accesses from the right femoral vein and left femoral artery. A right ventricular angiogram showed severely hypoplastic right ventricle including severely narrow infundibulum and no evidence of coronary sinusoids (Fig. 1). Therefore, radiofrequency perforation of the pulmonary valve and balloon valvuloplasty were performed successfully using a 6-mm VACS II at 5 atm (Video 1). The right ventricular systolic pressure reduced from 120 mmHg to 40 mmHg following the procedure, with a pressure gradient measured 30 mmHg between the right ventricular and pulmonary artery. Due to severe infundibular obstruction, we chose not to oversize the balloon (pulmonary valve annulus measuring 6.3 mm) to avoid iatrogenic valve regurgitation and proceeded with stenting of the patent ductus arteriosus to ensure a stable and sufficient source of pulmonary blood flow. Reference Aggarwal, Petit, Glatz, Goldstein and Qureshi1,Reference Promphan and Qureshi2 The patent ductus arteriosus is noted to be relatively straight, large, and arises from the proximal descending aorta. It would require a large stent from the arterial access which would have potentially led to pulmonary over circulation in a small infant. Reference Promphan and Qureshi2 To restrict flow across the ductal stent, we developed a novel technique with a plan to deploy from the venous access. We prepared a system including a 3.5-mm × 18-mm drug-eluting coronary stent over a wire which was housed in a 5F guide catheter. We advanced the system from the right femoral vein into the right atrium and ventricle, across the pulmonary valve, and crossing the patent ductus arteriosus into the descending aorta (Fig. 2). Approximately 1/3 of the stent and balloon were left inside the guide catheter while the balloon was inflated so that this stent portion would form a “funnel” and become a flow restrictor to the ductal stent (Video 2). The patient’s pulse oximetry was 90% on 30% FiO2 after the procedure, maintained adequate saturations during his hospital stay, and was discharged after 4 weeks. At his follow-up appointment at 11 months old, the patient was found to have severe pulmonary valve stenosis and the ductal stent became completely obstructed. He underwent a repeat balloon valvuloplasty at that time which resulted in mild to moderate pulmonary stenosis and regurgitation. He was doing well, was 2 years old at the time of this manuscript, without activity intolerance, and his pulse oximetry was 94% on room air.

Figure 1. Right ventriculogram demonstrating severe right ventricular hypoplasia on the antero-posterior (a) and lateral projection (b –d).
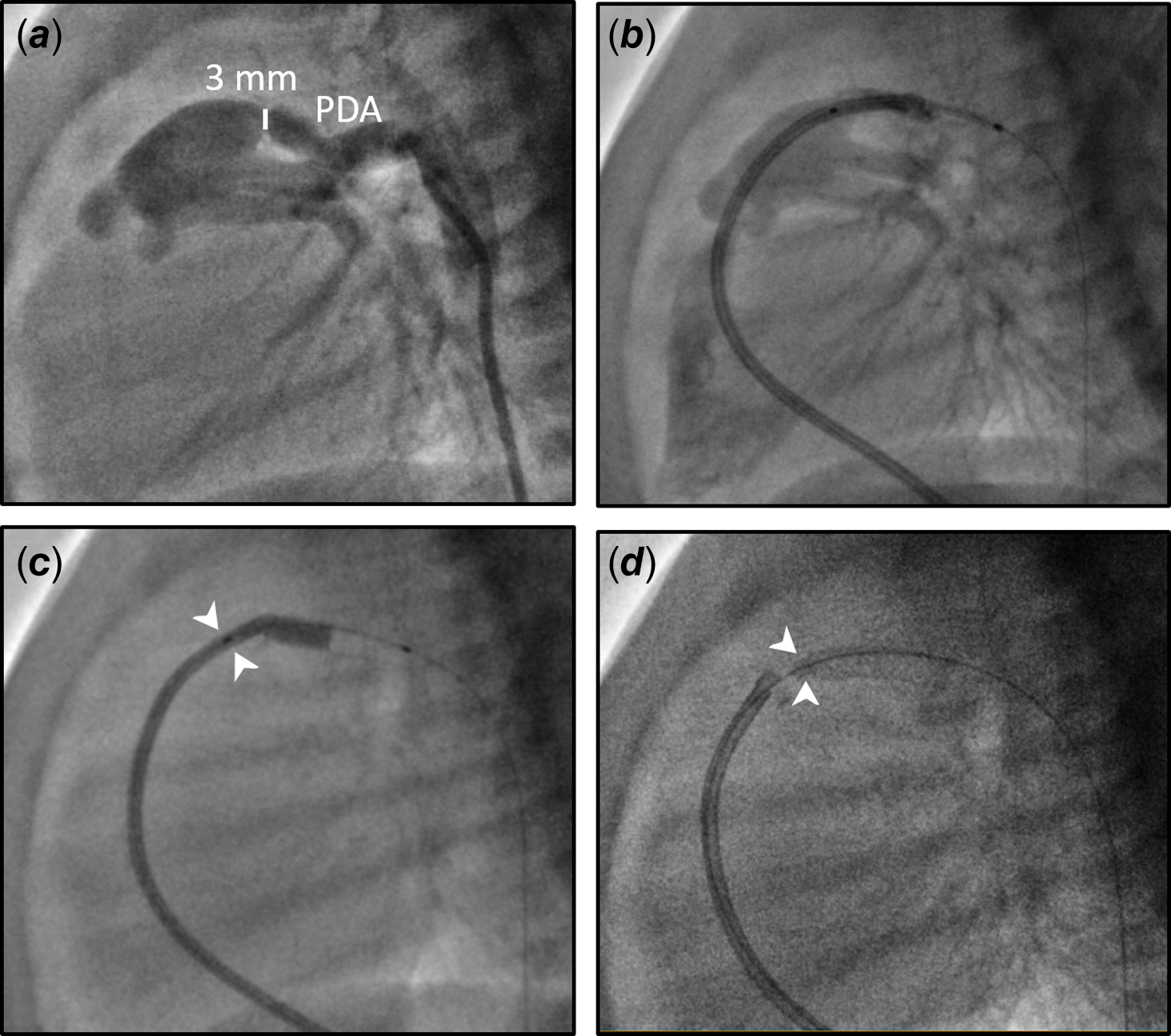
Figure 2. Size and shape of the patent ductus arteriosus (PDA) and novel technique to create a flow restrictor. a: Lateral view of the PDA (smallest diameter 3 mm), b: stent over a wire was advanced in a 5 Fr guide catheter across the PDA, c: 1/3 of stent remained within guide catheter during balloon inflation (white arrowheads), d: distal ductal stent with a funnel acting as a flow restrictor (white arrowheads).
To our knowledge, this was the first report utilising this technique to create a flow restrictor for a ductal stent in a small infant. The final pulmonary blood flow from ductal flow depends on the stent diameter and patent ductus arteriosus length. Reference Aggarwal, Petit, Glatz, Goldstein and Qureshi1,Reference Promphan and Qureshi2 The need to restrict ductal flow may not be easily identified during the procedure, although it should always be considered in small infant. In this case, a successful valve perforation and the straight patent ductus arteriosus type allowed for this technique to be feasible. We anticipated the stent “funnel” size to be approximately 1.92 mm (the internal diameter of the 5F guide catheter), which was estimated to provide an appropriate amount of additional pulmonary blood flow. Alternatively, a large stent could be placed from the arterial side with subsequent placement of a smaller stent within the first stent to restrict ductal flow, although it would increase cost and may arguably be more technically difficult. Reference Demir, Cools and Gewillig4
In conclusion, we presented a novel technique in which ductal stent flow restrictor was achieved in a unique scenario of a premature infant with pulmonary atresia and intact ventricular septum. We believe that the technique helped our patient have a balanced circulation immediately after the procedures and can be considered for other patients with similar anatomic substrates in the future.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S1047951124000258.





