Introduction
Congenital left ventricle to coronary sinus fistulas are exceptionally rare, and only a few case reports of this condition exist. These fistulas may isolated or occur in conjunction with other CHDs. Reference Aly, Singh and Shah1,Reference Shah, Teague, Lu, Dorfman, Kazerooni and Agarwal2 In this case report, we describe an 18-month-old boy who presented to our centre with a diagnosis of mitral valve insufficiency and a coronary sinus type atrial septal defect. Upon echocardiographic evaluation, he was found to have a rare congenital left ventricle to coronary sinus fistula. Echocardiographic examination established the diagnosis. Contrary to initial suspicions, mitral regurgitation and atrial septal defect were not present. Instead, the echocardiogram revealed a left ventricular to coronary sinus fistula accompanied by dilation of the coronary sinus. These findings were corroborated by CT angiography. The fistula was then successfully occluded using a transcatheter method. Follow-up assessments at 32 months post-procedure showed no complications and no evidence of significant shunting on echocardiography.
Case presentation
We report the case of an 18-month-old male, born full-term at 40 weeks without neonatal complications. At admission, his weight was 12 kg. The patient was acyanotic and asymptomatic. Cardiac examination revealed a prominent 3/6 systolic ejection murmur; however, there were no signs of heart failure or organomegaly, and weight gain was normal. Chest radiography indicated slight cardiomegaly, while the electrocardiogram showed sinus rhythm with a normal axis and no ST or T wave changes. A 2D and Doppler echocardiogram identified a dilated coronary sinus due to a left ventricular to coronary sinus fistula, with a jet waist measuring 3 mm and velocity around 4 m/sec. The pressure gradient across the fistula was noted at 60 mm Hg (Figure 1). Additionally, moderate right atrial enlargement and right ventricular enlargement were observed, with an Left Ventricular Ejection Fraction of 60%. Pre-procedural CT angiography provided a detailed anatomical evaluation, showing a width of 3 mm and a length of 4 mm (Figure 2). Catheterization revealed normal right atrial pressure and pulmonary artery pressures of 30/15 mmHg (mean 22 mmHg). Oxygen saturation levels indicated a significant left-to-right shunt with Qp/Qs of 1:1.5. Two catheterisation strategies, antegrade and retrograde, were evaluated for the occlusion procedure. Due to the intricate anatomical structure and the demanding wire-snaring location, the antegrade approach was favoured. This strategy facilitated traversal through the defect from the left ventricle to the coronary sinus, with successful snaring executed in the right atrium. In contrast, utilizing the retrograde approach would have necessitated wire snaring within the left ventricle, which was anticipated to be more challenging than the antegrade method. To close this fistula, a 4 French pig-tail catheter, which had been appropriately sized by trimming its tip, was first inserted into the left ventricle via the femoral artery. Subsequently, a 0.018-inch guidewire was used to traverse the fistula, and the wire was snared in the right atrium. After successfully navigating the fistula pathway, it was occluded using a Piccolo device (5 mm × 4 mm). The selection of this device was due to its optimal flexibility and the ability to conform to the anatomical configuration of the lesion. To ensure precise placement and maximum stability, the first disc of the device was positioned in the left ventricle, the body of the device within the fistula, and the final disc in the coronary sinus. Given the enlarged and dilated nature of the coronary sinus, positioning the edge of the device at the base of the coronary sinus did not interfere with the sinus pathway (Figure 3). Serial echocardiographic follow-ups before discharge and at 1, 6, 12, 24, and 32 months post-procedure showed minimal residual shunt initially, which resolved completely in subsequent studies with no shunt present at the fistula site (Figure 4). A 24-hour Holter monitor post-discharge confirmed normal sinus rhythm without ectopic beats or arrhythmias.

Figure 1. (a) The four-chamber view on the 2D echocardiogram reveals a dilated coronary sinus. (b,c) apical four-chamber view with colour Doppler showing the turbulent flow from the left ventricular (LV) to coronary sinus (CS). (d) A parasternal long-axis view with colour Doppler showing the fistula flow from the posterior-inferior aspect of the LV free wall to the CS. 2D, two-dimensional; CF, colour flow Doppler.
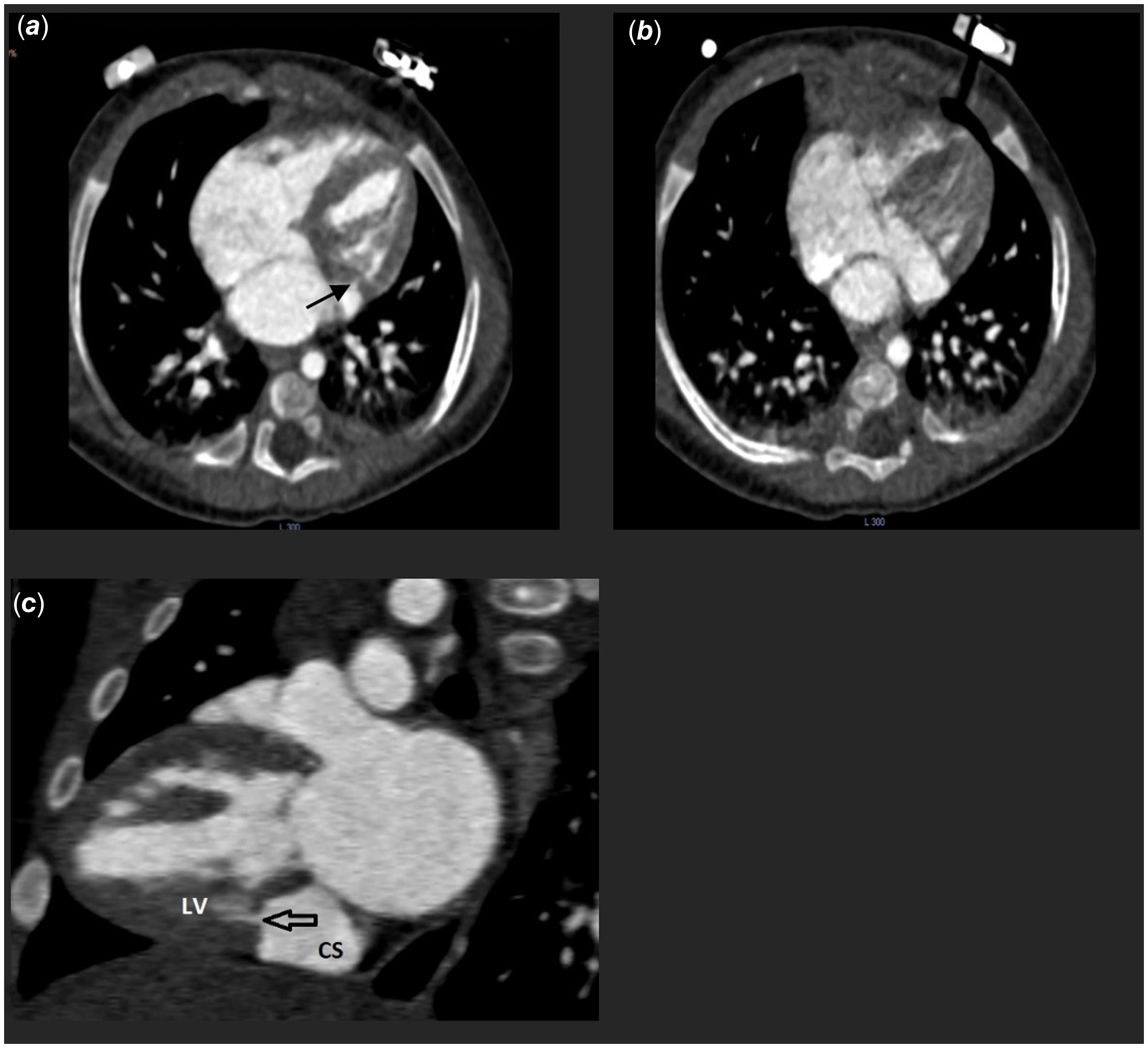
Figure 2. (a, b) An axial plane showing a dilated coronary sinus with a connection to the left ventricle (c) In the sagittal plane demonstrated the insertion of a fistula into the coronary sinus.
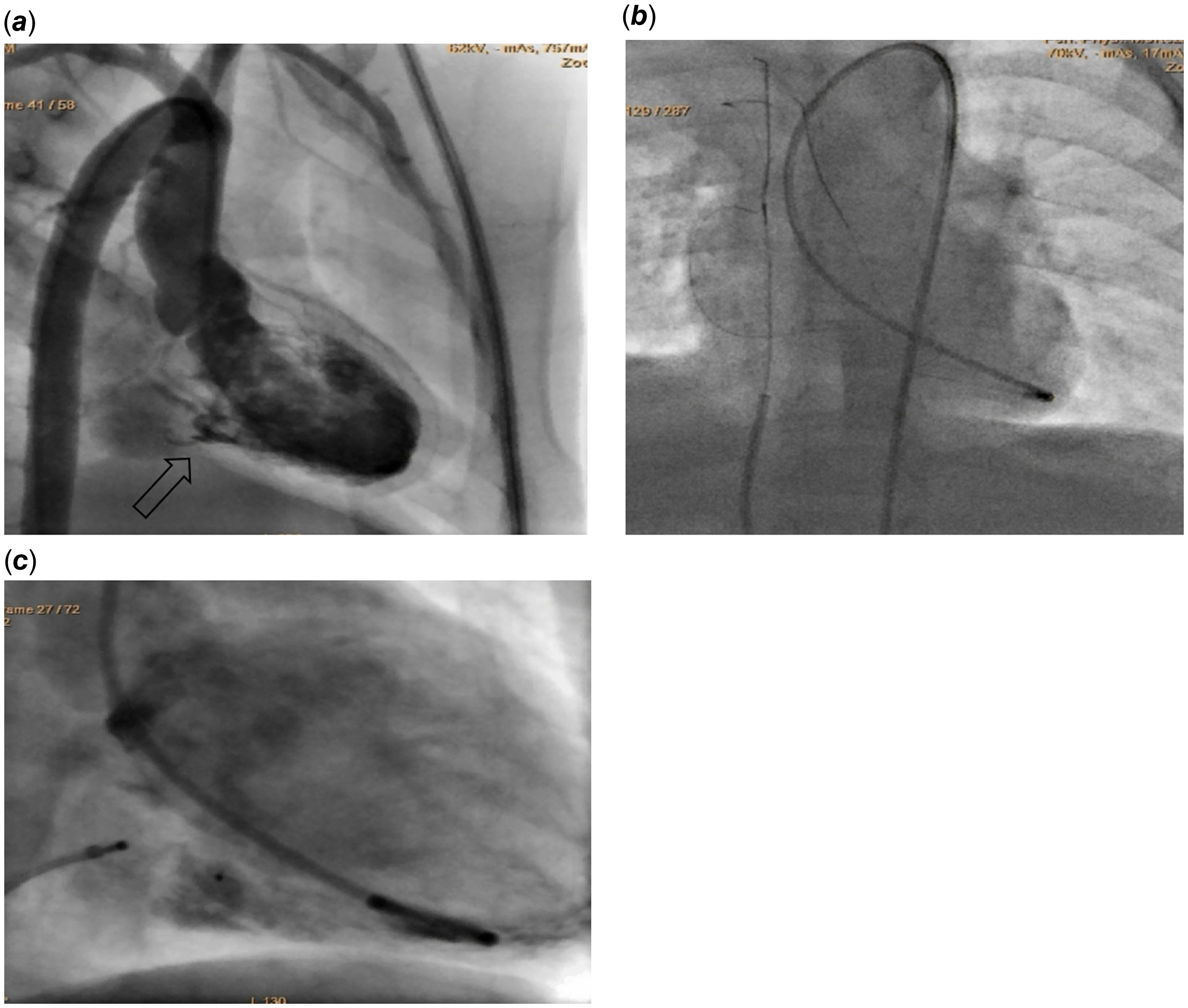
Figure 3. (a) Cineangiogram image, captured in the Right Anterior Oblique (RAO) view, demonstrates an injection that reveals a dilated coronary sinus in relation to the left ventricle (LV) (b) catheter was introduced via the right femoral artery to the LV, and a 0.018-inch guidewire was inserted into the coronary sinus, extending from it into the superior vena cava (SVC) where the guidewire was subsequently snared (c) Device adjustment and successful closure of a defect.
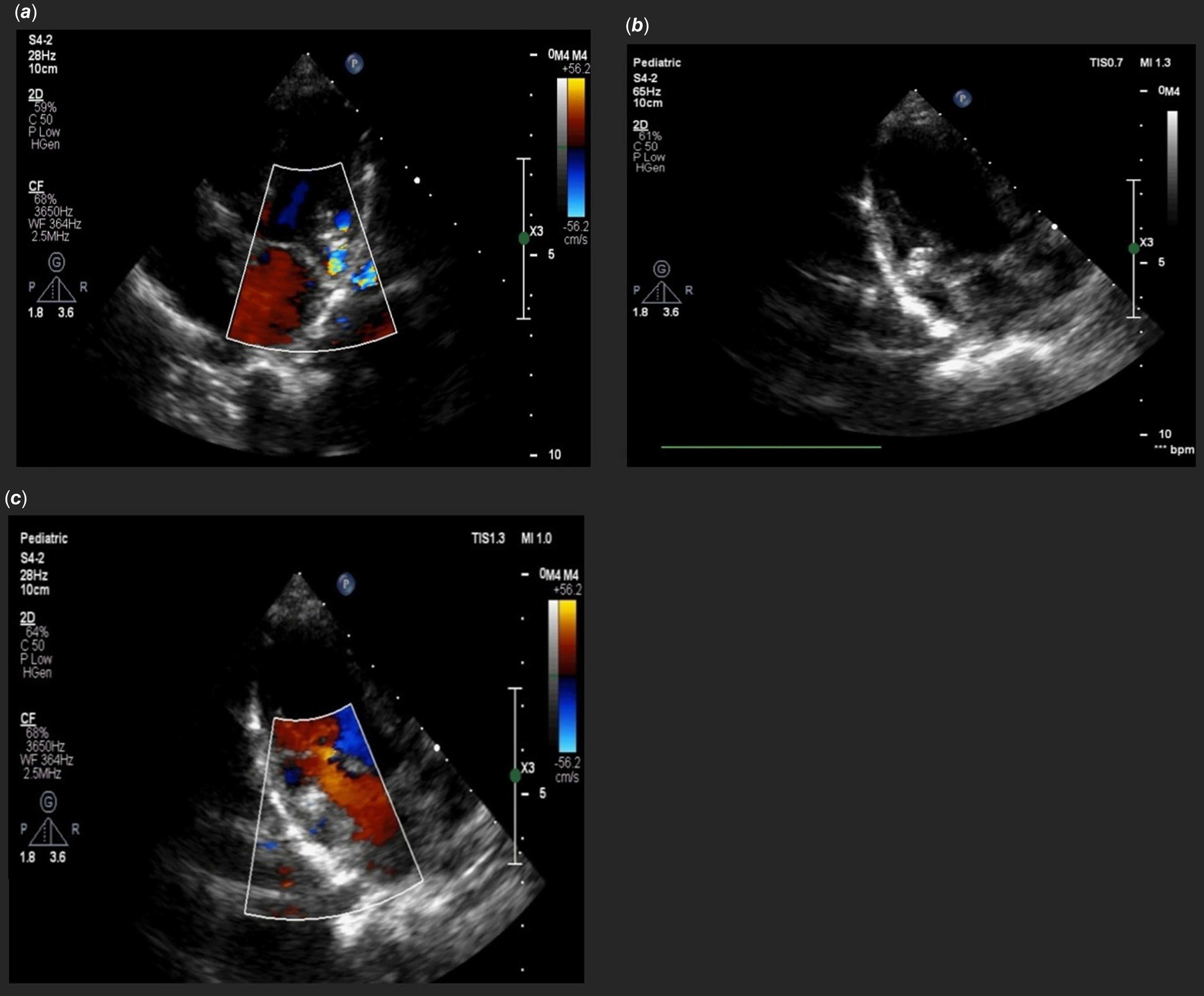
Figure 4. (a, b) Apical four-chamber view showed device in 2D and colour Doppler that revel no residual shunt from left ventricular to coronary sinus was remain (c) parasternal long-axis view with colour Doppler with no significant residual shunt. 2D, two-dimensional; CF, colour flow Doppler.
Discussion
The embryological explanation for left ventricle to coronary sinus fistula remains unclear. According to the separate embryological origins of the coronary sinus and the left ventricle, a connection between these two anatomical structures at the embryonic stage is theoretically unjustifiable. One of the agreed-upon justifications for describing this structural disorder is congenital weakness of mitral fibrous annulus and disjunction between the left ventricular musculature and the left atrium–mitral valve region was noted. Reference Baruah, Kumar, Reddy and Babu3
Complications following mitral valve replacement, post-catheter ablation procedures, and myocardial infarction are among the secondary causes associated with this disorder. Reference Mackie and Clements4–Reference Nayak and Victor7 One of the initial classifications of coronary sinus anomalies was proposed by Mantini et al., which was later refined by Shah et al. They suggested that a high-pressure coronary sinus fistula could result not only from a coronary artery to coronary sinus fistula but also from an left ventricular-to-coronary sinus fistula. Reference Shah, Teague, Lu, Dorfman, Kazerooni and Agarwal2,Reference Mantini, GRONDIN, LILLEHEI and EDWARDS8 One of the earliest documented cases of this congenital anomaly was reported by Gnanapragasam et al. in 1989. Reference Gnanapragasam, Houston and Lilley9 In our comprehensive review of the case reports registered to date, we identified two cases of isolated (left ventricle) to coronary sinus fistula. Additionally, three reports described patients with complex cardiac anomalies, including transposition of the great arteries with a ventricular septal defect, and a secundum atrial septal defect concomitant with a sub-mitral aneurysm communicating with the coronary sinus. Reference Aly, Singh and Shah1,Reference Gnanapragasam, Houston and Lilley9–Reference Méot, Bajolle and Bonnet12
Successful surgical approaches for the closure of this defect have been reported, with patch closure yielding good short-term results and minimal postoperative morbidity. Reference McGARRY, Stark and Macartney13 Reports about the successful closure of left ventricular to coronary sinus fistula by the transcatheter method have been reported through the coronary sinus approach. Reference Méot, Bajolle and Bonnet12, Reference Fontes Pedra, Pedra, Jatene Bosisio and Jatene14 Given the intricate and challenging anatomical access to the left ventricle to coronary sinus fistula, and considering the ease of snaring from the right atrium side, the retrograde approach emerges as a viable option. This is particularly advantageous in paediatric patients with low birth weight. Some studies also suggest that asymptomatic patients with no significant shunt can be managed with non-invasive monitoring. Periodic follow-ups, such as echocardiography and 24-hour Holter monitoring, are considered acceptable approaches. Reference Aly, Singh and Shah1 Additionally, in the case report by Minocha, Prashant K., et al., an left ventricular to coronary sinus fistula was noted in conjunction with Wolff–Parkinson–White syndrome. Alongside the closure of this defect, the patient’s aberrant pathway was ablated. Consequently, it is advised to employ a 24-hour Holter monitor to screen for arrhythmias during the follow-up of these patients. Reference Minocha, Saharan, Chun, Presti, Cecchin and Argilla15







