Airway obstruction is seen in 1%–2% of patients with CHD and can pose difficulties in their management. Reference McLaren, Elliott and Roebuck1 The associated symptoms of airway obstruction are non-specific and include wheezing, stridor, frequent upper respiratory tract infections, pneumonia, atelectasis, and feeding intolerance, which are also commonly seen in patients with CHD. It is therefore crucial to have a high index of suspicion for airway compression when evaluating patients with CHD and to have knowledge of these symptoms both pre- and post-operatively to rapidly identify and manage airway compromise. Reference McLaren, Elliott and Roebuck1
Airway pathology in patients with CHD can occur secondary to the anomalous relationship between the tracheobronchial tree and vascular structures, extrinsic compression of the airway by enlarged cardiac and/or pulmonary vascular structures, or an intrinsic airway abnormality (primary tracheo/bronchomalacia). While vascular rings are the most common cardiac congenital anomaly associated with airway compression, Reference McLaren, Elliott and Roebuck1 here we investigated other aetiologies of airway obstruction in patients with conotruncal abnormalities. Conotruncal anomalies are defined as malformations of the cardiac outflow tracts due to either a disturbance in the outflow tract of the embryonic heart, impaired development of the branchial arch and arteries, or both.
The aim of this study was to identify airway obstruction in patients with conotruncal anomalies observed by CT obtained for delineation of vascular anatomy prior to surgical intervention. The aetiology, location, and severity of airway obstruction were evaluated and associated with clinical presentations.
Materials and methods
Study design and patients
The Institutional Review Board approved the study protocol, and the requirement for informed patient consent was waived due to the retrospective nature of the study.
Our surgical database was reviewed to identify the total number of infants with the following conotruncal abnormalities as the primary diagnosis during the study period: double outlet right ventricle, tetralogy of Fallot, transposition of the great arteries, truncus arteriosus, and anomalous origin of the right pulmonary artery. A search of the cardiac CT database identified patients with complex conotruncal anomalies who underwent pre-operative cardiac CT for the assessment of vascular anatomy between 2014 and 2018 in order to help guide surgical planning. Complexity was defined as having abnormal or unexpected vascular anatomy prompting further investigation. CT images, echocardiographic reports, and clinical information obtained during their hospital admission were reviewed. Any interventions administered due to the presence of respiratory distress upon arrival to the cardiac ICU or prior to surgical repair were noted. Chromosomal microarray results were also documented. Patients with vascular rings and absent pulmonary valve syndrome were excluded from this study.
CT scanning
High-pitch spiral CT of the chest was performed utilising a 128-detector dual-source CT scanner (Somatom Definition FLASH, Siemens Healthcare, Forcheim, Germany; tube rotation time 280 m/sec, temporal resolution 75 m/sec, collimation 2 × 128 × 0.6 mm). Dose reduction strategies were utilised including low kVp selection, automatic tube current modulation, and iterative reconstruction. Post-processing techniques including multiplanar reconstruction, maximum intensity projection, and volume rendering were performed. Iodinated contrast (Iohexol 300 or 350) was administered utilising a power injector with biphasic injection (contrast followed by saline). The standard contrast medium dose was 2 mL/kg, and the maximum dose was 3 mL/kg. The dose length product was retrieved from the dose report from the CT scanner. Total effective dose was calculated using a commercially available dose management programme (Radimetrics, Bayer Healthcare, Berlin, Germany).
Imaging review
All CT reports were reviewed followed by extensive retrospective review of the source data by both a staff attending radiologist and cardiologist. Images were reviewed for: (i) dilatation of the ascending aorta; (ii) sidedness of the aortic arch; (iii) morphology of the arch; (iv) interaortic distance, that is, the distance between the ascending aorta and thoracic descending aorta at the same level in the axial plane Reference Lee, Kim and Baek2 ; (v) presence of a patent ductus arteriosus and its morphologic features; (vi) surrounding vascular structures causing compression to the airway, with particular attention to the branch pulmonary arteries, the innominate artery, and aortopulmonary collaterals; (vii) severity of obstruction along the tracheobronchial tree, which was classified into either mild or severe; mild obstruction was defined by the presence of a mostly patent airway with slight compression, while severe obstruction was defined by the presence of obliteration or near obliteration of the airway; (viii) the anatomical level of airway obstruction of the tracheobronchial tree: trachea, left main stem bronchus, or right main stem bronchus; and (ix) evidence of intrinsic airway abnormalities.
Statistical analysis
Descriptive data are presented as median (interquartile range) for continuous variables and as n (%) for categorical data. Chi-square tests were used to compare differences in proportions between the outcome groups and the Mann–Whitney U-test was used to test for differences in continuous variable between groups. All statistical tests were two-tailed, and a p-value less than 0.05 was considered significant. Analyses were performed in SPSS (version 23.0, IBM Corp, Armonk, NY).
Results
Clinical characteristics and occurrence of airway obstruction
There was a total of 199 infants during the study period with conotruncal abnormalities at our institution. Specifically, there were 32 infants with double outlet right ventricle, 94 with tetralogy of Fallot, 56 with transposition of the great arteries, 15 with truncus arteriosus, and 2 with aortic origin of the right pulmonary artery. Forty-six patients who met the inclusion criteria underwent CT scan for delineation of vascular anatomy. The patient demographics are presented in Table 1. Airway obstruction was reported in 19/46 patients (41%), of whom 13/19 (68%) were classified as having severe compression. A right aortic arch was noted in 8/19 (42%) patients in the airway obstruction group compared to 3/27 (11%) in the non-obstructed group (p < 0.05).
Table 1. Patient demographics

CMA = chromosomal microarray; RPA = right pulmonary artery.
The anatomic structures noted to cause obstruction and patients with intrinsic obstruction are detailed in Table 2. Compression was most often noted secondary to the transverse aortic arch (n = 5; Fig 1) and patent ductus arteriosus (n = 5; Fig 2). Of patients with compression by the transverse arch, the anatomy of the transverse arch was diverse, with severe dilation, abnormal orientation, and reverse C-shape all noted (Fig 1). Those with obstruction due to a patent ductus arteriosus had an abnormally configured ductus, either markedly prominent (associated with an interrupted aortic arch in two patients) or markedly tortuous and, in one patient, associated with bilateral ductus. A short interaortic space resulted in airway compression in two patients (Fig 3). Intrinsic airway obstruction was noted in four patients (Fig 4). Additionally, airway obstruction caused by the right pulmonary artery was noted in one patient, by the configuration of the left pulmonary artery in another patient, and by the innominate artery in a third patient (Fig 5). Two cases showed multiple vascular structures causing obstruction. The most common site of obstruction was at the level of the trachea (n = 12) followed by the left mainstem bronchus (n = 10) and then the right mainstem bronchus (n = 8). However, 8/19 (45%) patients demonstrated multilevel obstruction along the tracheobronchial tree. Table 3 outlines all patients in the airway obstruction group and includes details regarding necessary respiratory support as well as their most current clinical statuses. All but one mortality were in patients with severe airway obstruction. Respiratory failure and cardiac arrest were among the most likely causes of demise in this group. All figures can be matched to the patient details by case number.
Table 2. Aetiology of airway obstruction

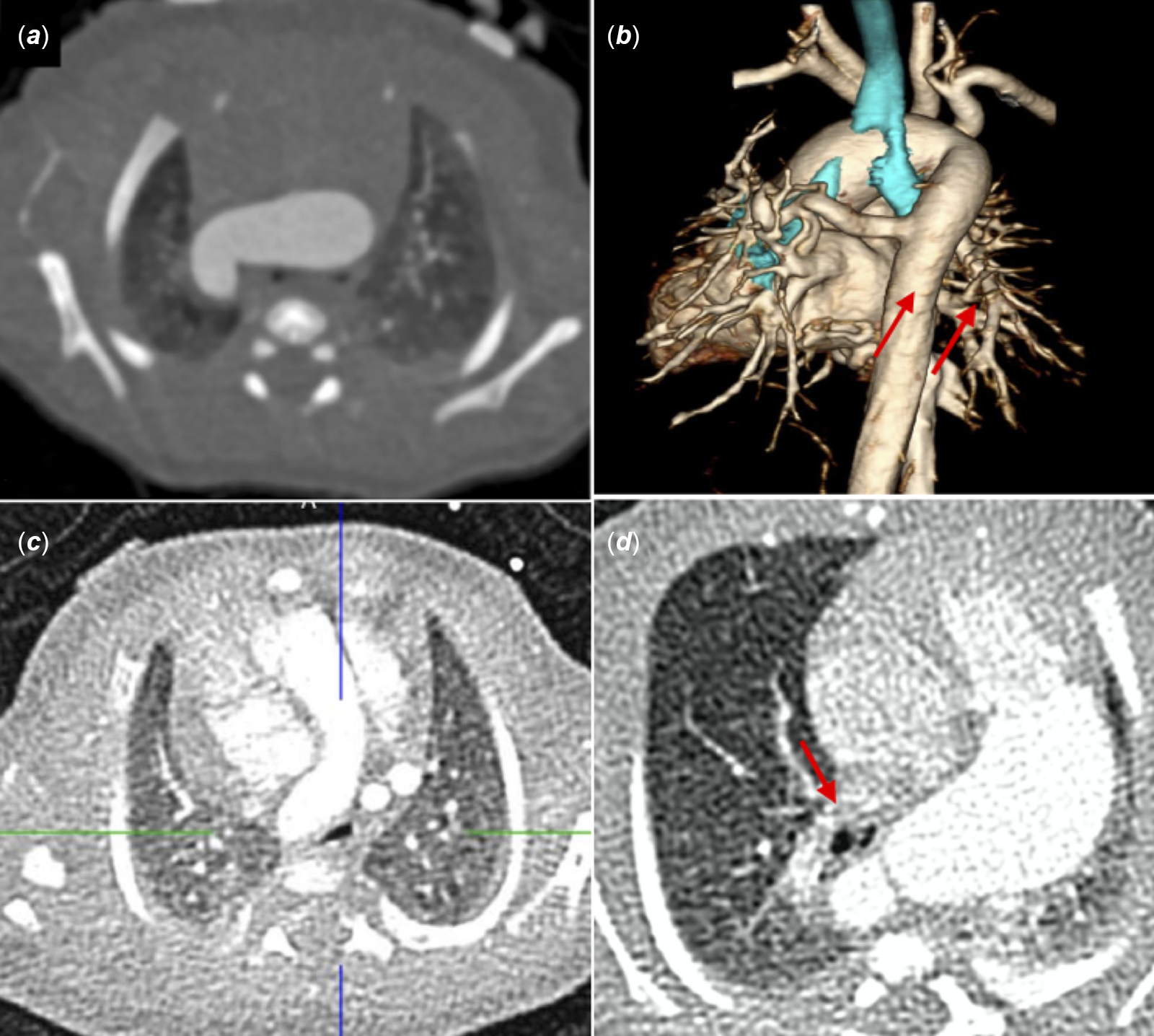
Figure 1. Airway obstruction by aortic arch. A case of tetralogy of Fallot, pulmonary atresia, and right aortic arch. There is complete obliteration of the left mainstem bronchus secondary to the horizontal orientation of the dilated transverse arch. The right mainstem bronchus is also obstructed (a, b). A case of double-outlet right ventricle, unbalanced atrioventricular canal, and long segment pulmonary atresia. Compression of trachea by “reverse C-shaped” right aortic arch. (c). A case of tetralogy of Fallot, pulmonary atresia and major aortopulmonary collaterals (MAPCAs). Marked compression of distal trachea and proximal left stem bronchus (arrow) by abnormal orientation of the dilated left aortic arch (d).

Figure 2. Airway obstruction by patent ductus arteriosus. A case of truncus arteriosus and interrupted aortic arch. Mild compression of left main bronchus by a dilated ductus arteriosus (arrows) shown in 3D (a) and straight coronal plane (b). A case of tetralogy of Fallot with pulmonary atresia. Marked compression of the left main bronchus by the tortuous ductus arteriosus (arrows) shown in 3D (c) and straight coronal plane (d).

Figure 3. Airway obstruction by short interaortic space. Case of tetralogy of Fallot with pulmonary atresia and major aortopulmonary collaterals. Note the very short distance between the ascending aorta and descending aorta (arrow) resulting in severe compression of right main bronchus.

Figure 4. Intrinsic airway obstruction. A case of transposition of the great arteries, small ventricular septal defect and pulmonary stenosis. There is mild indentation in mid trachea (a) and right main stem bronchus is smaller than the left (b).

Figure 5. Airway obstruction by branch pulmonary arteries and aortopulmonary collaterals. A case of anomalous origin of the right pulmonary from the aorta (arrow) compressing the distal left main bronchus (a). A case of double-outlet right ventricle. The left main stem bronchus is compressed by left pulmonary artery (arrow) (b). A case of tetralogy of Fallot with pulmonary atresia and major aortopulmonary collaterals. The left main stem bronchus is compressed by a collateral (arrow) (c).
Table 3. Pre-surgical findings and post-surgical outcomes in infants with conotruncal abnormalities with airway obstruction

AOV = aortic valve; AVV = atrioventricular valve; AVC = atrioventricular canal; BAS = balloon atrial septostomy; B/L = bilateral; CAVC = complete atrioventricular canal; CS = coronary sinus; DKS = Damus-Kaye-Stansel; DORV = double outlet right ventricle; ECMO = extracorporeal membrane oxygenation; IAA = interrupted aortic arch; IVC = inferior vena cava; LPA = left pulmonary artery; LSVC = left superior vena cava; LV = left ventricle; MAPCAs = Major aortopulmonary collateral arteries; MPA = main pulmonary artery; MV = Mitral valve; PA = pulmonary artery; PAPVR = partial anomalous pulmonary venous return; PDA = patent ductus arteriosus; PS = pulmonary stenosis; RAA = right aortic arch; RV = right ventricle; RVOT = right ventricular outflow tract; TAP- transannular patch; TGA = transposition of the great arteries; TOF = Tetralogy of Fallot; VSD- ventricular septal defect
Respiratory interventions
Respiratory support at birth, from oxygen supplementation to intubation, was required in 10/19 (53%) of the airway obstruction group versus 13/27 (48%) of the non-obstructed group (p = 0.699). With respect to mortality, 5/19 (26%) patients in the airway obstruction group versus 2/27 (7%) patients in the non-obstructed group (p = 0.078) died (Table 1). Nine of the 46 (20%) patients were intubated at the time the CT scan was performed.
Genetics
Within this patient subset, 25 (54%) patients had a normal chromosomal microarray, seven (15%) patients had an abnormal chromosomal microarray with a variant of unknown significance, and 3 (6%) patients had a specific syndrome. Five (11%) patients had 22q11.2 deletion syndrome (Table 1).
CT scans
The median age at the time of pre-operative CT scanning was 5 days (range 1–262 days). The average radiation dose length product for the protocol utilised for scanning was 18 mGy cm (range 5–66 mGy cm). The average effective dose was 2.3 millisievert (mSv; range 0.8–10 mSv).
Discussion
This study identified 46 patients with complex conotruncal anomalies who underwent pre-operative CT scans. This class of defects includes truncus arteriosus, tetralogy of Fallot, transposition of the great arteries, interrupted aortic arch, and double outlet right (or left) ventricle. Airway obstruction was noted in 41% of patients, with over half classified as having moderate to severe compression. This is particularly high considering that the indication for the CT was evaluation of the underlying heart defect and that airway obstruction was not suspected. Furthermore, airway obstruction would have remained undetected if the CT scans had not been not performed. The majority of patients had extrinsic compression at the level of the transverse aortic arch, with a significant predominance of compression in patients with a right aortic arch. Approximately 20%–25% of patients with tetralogy of Fallot and 21%–36% of patients with truncus arteriosus have a right aortic arch, making this finding particularly pertinent. Reference Allen, Driscoll, Shaddy and Feltes3 Understanding this relationship may be helpful in identifying airway obstruction in this patient subset.
Airway obstruction related to a dilated aorta has been described in several case reports and case series, Reference Capitanio, Wolfson, Faerber, Williams and Balsara4–Reference McElhinney, Reddy, Pian, Moore and Hanley9 with the underlying heart diseases including truncus arteriosus, tetralogy of Fallot, isolated ventricular septal defect, and cervical arch. Similarly, in our study, airway obstruction by the aorta was the most common aetiology. Seven of 19 patients had airway compression secondary to an abnormal transverse arch, most of whom had very unique and polymorphic aortic anatomy. Although several characteristics of the aortic arch including the sidedness, dilatation, and tortuosity can be readily assessed by transthoracic echocardiography, the three-dimensional orientation of the arch is much better defined in CT images. Furthermore, the effect of such arch characteristics on the airway is undetectable by echo but very well demonstrated by CT.
Three of 19 patients had airway obstruction due to a patent ductus arteriosus, which has not previously been reported. Our study only assessed pre-operative CT scans; therefore, we cannot comment on whether ductal closure influenced the degree of post-operative airway obstruction or whether it resolved after ductal closure.
Of particular note, though not found to be statistically significant, there was a trend towards a higher mortality rate in patients with airway obstruction. Corno et al Reference Corno, Giamberti and Giannico5 previously noted significant morbidity and mortality in 12 infants with airway compression immediately following palliation or repair for CHD. Other case reports and case series of airway obstruction have also reported high morbidity and mortality rates. Reference Capitanio, Wolfson, Faerber, Williams and Balsara4,Reference Li, Wang and Chen8,Reference An, Choi and Kwon10,Reference Habbema, Losekoot and Becker11
Various imaging modalities have been used to identify the source of airway obstruction in patients with vascular anomalies pre- and post-operatively to better understand the anatomy and formulate a surgical plan, including but not limited to radiographs, flexible bronchoscopy, fiberoptic endoscopy, tracheography, and cardiac MRI. Reference Corno, Giamberti and Giannico5,Reference Habbema, Losekoot and Becker11–Reference Robotin, Bruniaux and Serraf15 However, these procedures have associated risks including bleeding, bronchospasm, and hypoxia requiring emergency intubation, which may not be well tolerated in an already vulnerable patient population. Reference Habbema, Losekoot and Becker11 In some cases, multiple techniques have been used to help delineate obstructive anatomy, but it is important to note that each additional procedure has an additive risk and cost to the patient without definitively providing more information. CT, however, is widely utilised, is widely available, quick, non-invasive, and fairly inexpensive, and can be conducted using no or minimal sedation. We show that cardiac CT provides excellent visualisation of airway obstruction as it relates to surrounding cardiovascular structures. Our CT scanning technique is a rapid, non-invasive means to image patients while limiting radiation exposure.
Previously conducted epidemiological studies have suggested that a genetic cause can be identified in many patients with CHD. Conotruncal malformations specifically account for 70% of heart defects associated with 22q11.2 deletion and have been associated with CHARGE syndrome, Adams–Oliver syndrome, and Goldenhar syndrome amongst others. Though there were numerous patients in our study with genetic abnormalities, there was no significant difference in the presence of genetic abnormalities in patients with or without airway obstruction. Reference Pierpont, Brueckner and Chung16
Limitations of this study include its small population size, retrospective nature, interobserver reliability, and CT technique. The sample size was relatively small, as we were limited to examining retrospective data on patients whose evaluation was reliant on CT scans for improved delineation of complex vascular anatomy. Since the indication for CT was clarification of complex vascular anatomy, these studies were not tailored to critically evaluate airway anatomy and therefore not all reports included pertinent airway information. Of note, this also may contribute to a general underestimation in the incidence of airway obstruction. Additionally, the degree of severity of airway compression reported may be regarded as subjective, as no definitive quantitative measurements were made at the time that the scans were performed. Both a radiologist and cardiologist examined the source data to help ensure interobserver reliability. Motion artefact from respiration and cardiac pulsation is an unavoidable limitation of cardiac CT. Spiral CT with 3D reconstruction can be particularly useful when evaluating compression of the central airways in patients with CHD, and multi-slice CT has helped to improve image quality and minimise artefact. Despite this, over- and underestimating the degree of airway stenosis is a known limitation of this modality. Reference Choo, Lee and Ban17 Though our CT scan technique has been designed to provide optimal imaging while limiting radiation exposure, our current technology cannot account for respiratory variation and therefore cannot achieve a truly dynamic evaluation of the airway. We therefore cannot be certain whether our images captured the largest or smallest airway diameters. A more advanced system would need to be utilised to guarantee such accuracy. Of note, patients who were intubated during these studies and had positive pressure ventilation may have demonstrated false patency of their natural airway.
Future studies of particular interest would include a multi-centre analysis of patients with complex conotruncal anomalies to further analyse incidence and the post-surgical airway anatomy and incidence of airway obstruction.
Conclusions
Airway obstruction in complex conotruncal abnormalities is much more prevalent and severe than previously reported. The ability to understand airway anomalies prior to surgical repair may help anticipate the need for additional interventions at the time of palliation, such as aortopexy or airway reconstruction. The presence of airway obstruction may help predict outcome in high-risk patients and prepare clinicians and families for future complications and challenges.
Acknowledgements
None.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflicts of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008, and have been approved by the institutional committees.











