Introduction
One of the challenges of geriatric medicine is prescribing safe, effective medications with few side effects. Many medications which are safe and effective in younger patients (i.e., < 65 years of age) are not the best option or should be avoided in older adults (i.e., ≥ 65 years of age) because of the change in pharmacokinetics (i.e., absorption, distribution, metabolism, and excretion) and pharmacodynamics (the physiologic effects of the drug) with age (Beers, Baran, & Frenia, Reference Beers, Baran and Frenia2000; Chang & Chan, Reference Chang and Chan2010; Reeve, Trenaman, Rockwood, & Hilmer, Reference Reeve, Trenaman, Rockwood and Hilmer2017).
Polypharmacy is variously defined as high numbers of medications (e.g., concurrent use of five or more medications), use of more drugs than are clinically indicated, and/or use of inappropriate medications (Masnoon, Shakib, Kalisch-Ellett, & Caughey, Reference Masnoon, Shakib, Kalisch-Ellett and Caughey2017). In 2016, nearly two thirds (65.7%) of older adults were prescribed 5 or more different drug classes, with more than one quarter (26.5%) being prescribed 10 or more different drug classes, and 8.4 per cent prescribed 15 or more drug classes (Canadian Institute for Health Information, 2018). The impact of polypharmacy on older adults is significant. Polypharmacy is associated with poor medication adherence, drug–drug interactions, medication errors, adverse drug events, and an increased incidence of falls, hip fractures, confusion, and delirium. Collectively, polypharmacy in older adults accounts for a significant percentage of potentially preventable emergency room visits and hospitalizations (von Buedingen et al., Reference von Buedingen, Hammer, Meid, Müller, Gerlach and Muth2018). Polypharmacy also increases the incidence of potentially inappropriate medication (PIM) use as defined by the American Geriatrics Society (AGS) (Jano & Aparasu, Reference Jano and Aparasu2007; Reason, Terner, Moses, Tipper, & Webster, Reference Reason, Terner, Moses, Tipper and Webster2011; Spinewine et al., Reference Spinewine, Schmader, Barber, Hughes, Lapane and Swine2007).
Avoiding the use of inappropriate medications and PIMs is essential to prevent both adverse drug effects in the elderly (e.g., morbidity, mortality) and increased health care expenses. Methods for identifying inappropriate and PIMs include implicit and explicit criteria. An implicit criterion depends on prescriber judgement; for example, assessing drug–drug interaction or therapeutics duplicate by comprehensive geriatric assessment (CGA), or by using the Medication Appropriateness Index (Hanlon & Schmader, Reference Hanlon and Schmader2013).
Explicit criteria identify high-risk drugs using a list of PIMs that have been identified through expert panel review and consider alternative medications (American Geriatrics Society 2012 Beers Criteria Update Expert Panel, 2012; Basger, Chen, & Moles, Reference Basger, Chen and Moles2008; Beers, Reference Beers1997; Beers et al., Reference Beers, Ouslander, Rollingher, Reuben, Brooks and Beck1991; Fick et al., Reference Fick, Cooper, Wade, Waller, Maclean and Beers2003, Reference Fick, Semla, Steinman, Beizer, Brandt and Dombrowski2019; Gallagher & O’Mahony, Reference Gallagher and O’Mahony2008; Laroche, Charmes, Bouthier, & Merle, Reference Laroche, Charmes, Bouthier and Merle2009; McLeod, Huang, Tamblyn, & Gayton, Reference McLeod, Huang, Tamblyn and Gayton1997; Rancourt et al., Reference Rancourt, Moisan, Baillargeon, Verreault, Laurin and Grégoire2004; Zhan et al., Reference Zhan, Sangl, Bierman, Miller, Friedman and Wickizer2001). Common examples include The Beers Criteria, the Screening Tool for Older Persons Prescribing (STOPP) criteria (O’Mahony et al., Reference O’Mahony, O’Sullivan, Byrne, O’Connor, Ryan and Gallagher2015), the Laroche list of PIMs (Laroche et al., Reference Laroche, Charmes, Bouthier and Merle2009) and the multiple country/region-specific FORTA (Fit fOR The Aged) List (Pazan, Weiss, Wehling, & FORTA, Reference Pazan, Weiss and Wehling2018). However, any criteria should take a multifactorial approach considering the patient as a whole, including his or her life expectancy, quality of life, essential medications, and avoiding drugs with a poorer benefit‐to‐risk ratio (Laroche et al., Reference Laroche, Charmes, Bouthier and Merle2009).
The Beers Criteria consist of a list of PIMs developed and published by Beers and colleagues for nursing home residents in Reference Beers, Ouslander, Rollingher, Reuben, Brooks and Beck1991. The criteria have subsequently been expanded and revised, lastly in 2019 to include all settings of geriatric care (Fick et al., Reference Fick, Semla, Steinman, Beizer, Brandt and Dombrowski2019). The use of medications on the PIM list have been found to be associated with poor health outcomes in the elderly, including confusion, falls, and increased mortality (Fick, Mion, Beers, & Waller, Reference Fick, Mion, Beers and Waller2008; Stockl, Le, Zhang, & Harada, Reference Stockl, Le, Zhang and Harada2010). PIM use is known to be associated with geriatric syndromes (Kucukdagli et al., Reference Kucukdagli, Bahat, Bay, Kilic, Oren and Turkmen2019). In addition to the significant side effects and increased need for medical attention, there also are significant system costs to PIM use. For example, PIM use affects patient morbidity and mortality rates and increases in hospitalization which, in turn, add significantly to health care costs (Jano & Aparasu, Reference Jano and Aparasu2007; Spinewine et al., Reference Spinewine, Schmader, Barber, Hughes, Lapane and Swine2007). Health care expenditure related to PIM use in the United States has been estimated at $7.2 billion and in Canada at $419 million (Canadian Institute for Health Information, 2018; Fu et al., Reference Fu, Jiang, Reeves, Fincham, Liu and Perri2007).
PIM use in older adults in Canada is highly prevalent. The Canadian Institute for Health Information (CIHI) reported that in 2016, nearly half of older adults (49.4%) had at least one prescription for a drug on the AGS Beers Criteria PIM list. Some 18.0 per cent of older adults had prescriptions for multiple drugs on the same Beers Criteria list, including 8.1 per cent who were chronic users of two or more different drugs (Canadian Institute for Health Information, 2018). PIM use in long-term care (LTC) in patients with advanced dementia further exacerbates PIM use. In one study by Holmes and colleagues, the researchers found that, of such patients, 29 per cent were taking a medication considered never to be appropriate (Holmes et al., Reference Holmes, Sachs, Shega, Hougham, Cox Hayley and Dale2008).
The 2014 Cochrane Systematic review by Patterson, Cadogan, Kerse, Cardwell, and Bradley (Reference Patterson, Cadogan, Kerse, Cardwell and Bradley2014) looked at the effectiveness of “pharmaceutical care" to improve the appropriate use of polypharmacy for older adults. The pharmaceutical care was commonly provided by pharmacists conducting medication reviews and working closely with other health care professionals in a variety of settings. The results indicated that there was a positive change in the appropriateness of medications prescribed in the intervention group as well as a decrease in the number of Beers PIM use per participants (Patterson et al., Reference Patterson, Cadogan, Kerse, Cardwell and Bradley2014). However, the update in 2018 did not find clinically significant improvement (Rankin et al., Reference Rankin, Cadogan, Patterson, Kerse, Cardwell, Bradley, Ryan and Hughes2018). In other studies, medication review by the pharmacist was shown to be effective in identifying PIM use in the elderly (Tommelein et al., Reference Tommelein, Mehuys, Van Tongelen, Petrovic, Somers and Colin2017), as well as in reducing polypharmacy on the acute inpatient ward (Nielsen, Honoré, Rasmussen, & Andersen, Reference Nielsen, Honoré, Rasmussen and Andersen2017), in the emergency room (Mogensen, Thisted, & Olsen, Reference Mogensen, Thisted and Olsen2012), and in community settings (Messerli, Blozik, Vriends, & Hersberger, Reference Messerli, Blozik, Vriends and Hersberger2016).
Much is known about the dangers of PIM use and pharmacist-led interventions to reduce PIM use. However, there are no studies to our knowledge that have studied the effect of geriatrician led CGAs on PIM use in community-dwelling older adults attending geriatric outpatient clinics at two points in time. Our patients frequently have geriatric syndromes and cognitive impairment. As such, our objective was to determine the prevalence of PIM use among community-dwelling patients referred for CGA at two different geriatric clinics at two points in time, and whether their use was addressed in the CGA.
Methods
Study Design
This study consisted of two cross-sectional retrospective chart reviews of patients ≥ 65 years of age seen in the outpatient geriatric clinics in two tertiary medical centres in Canada (Edmonton, Alberta) across two time periods. The clinics were geriatrician-led, often with resident involvement, with the CGA completed in one visit. Although one clinic did have access to a pharmacist, there were very few pharmacy notes in the charts reviewed. In 2014, we reviewed 200 randomly selected charts of patients who had CGAs between January 1, 2012 and December 31, 2013 at the Glenrose Rehabilitation Hospital (Glenrose). In 2019, we reviewed randomly selected charts of patients who had CGAs at the Misericordia Community Hospital (Misericordia) between January 1, 2016 and December 31, 2017. Charts were randomly selected from a list of eligible charts by using random numbers generated online (random.org). We included patients 65 years of age or older who had CGAs in the outpatient department of the Glenrose and Misericordia Hospitals. We excluded patients who were directly admitted to hospital as a result of the CGA. This was because recommendations were not made in clinic, as they would be taken over by the admitting physician. The ethics board of the University of Alberta approved both studies separately (Study ID Numbers Pro00043873 and Pro00082754).
The CGA collects history on all regular and as-needed prescribed and over-the-counter medications from at least two sources: a provincial electronic pharmacy list that includes a best possible medication history and from patients/caregivers who are advised to bring all medications to the appointment. The CGA additionally collects information on presenting problems, geriatric syndromes, co-morbidities, and family/social/functional/cognitive history, as well as the results from cognitive and physical examinations, to give a global picture of the patients’ medical, psychological, and social health and limitations in order to develop an overall plan for treatment and long‐term follow‐up (Rubenstein, Stuck, Siu, & Wieland, Reference Rubenstein, Stuck, Siu and Wieland1991). The CGAs were examined to extract demographics, reason for the referral, geriatric syndromes, past medial history, names and number of current medications, prevalence of PIM use, common PIMs used, and the corresponding recommendations (i.e., ordered to be stopped, tapered, decreased/increased, or reviewed [with patient refusing recommendations or to be reviewed at follow-up] collected from the dictated recommendations in the CGA). To determine whether medications were potentially inappropriate, we used the 2012 and 2015 AGS Beers Criteria for the chart reviews in 2014 and 2019, respectively, after reviewing the past medical history. Specifically, we used the medications listed Table 2 of the Beers criteria (PIM Use in Older Adults). We did not use Table 3 of the Beers criteria (PIM Use in Older Adults due to Drug-disease or Drug-Syndrome Interactions that May Exacerbate the Disease or Syndrome) because of the variability of the completeness of the medical history in the charts. Specifically, we used as a reference the Beers List of Medications to Avoid in Older Adults. The data were collected, using a standard data collection sheet, by two third-year medical residents training in care of the elderly, who were already qualified family physicians.
Statistical Analyses
Descriptive statistics (frequencies, percentages, means, medians, and standard deviations) were used to describe the sample and to characterize the PIM data.
Results
The results are presented from the chart reviews from the Glenrose Rehabilitation Hospital first, followed by the chart reviews from the Misericordia Community Hospital.
Patient Demographics and PIM Use
Glenrose Rehabilitation Hospital
The mean age of patients was 79.4 years (standard deviation [SD] = 7.7 years) with a predominance of females (60.5%). The median number of co-morbidities was seven. In 77.5 per cent of cases (155/200), the reason for referral was cognitive assessment. The average number of medications per patient was 9.0 (SD = 4.3). The prevalence of PIM use among patients was 45 per cent (90/200). Among the 90 patients who had PIM use, a total of 130 PIMs were identified, with some patients having more than one PIM (range: 1–4 PIMs per patient). The most frequent medications were zopiclone, clonazepam, lorazepam, amitriptyline, cyclobenzaprine, ibuprofen, and quetiapine. These seven drugs accounted for 61.5 per cent (80/130) of PIM use (see Table 1 and Appendix A).
Table 1. Characteristics of patients seen in the outpatient geriatric clinics at the Glenrose Rehabilitation Hospital and Misericordia Community Hospital
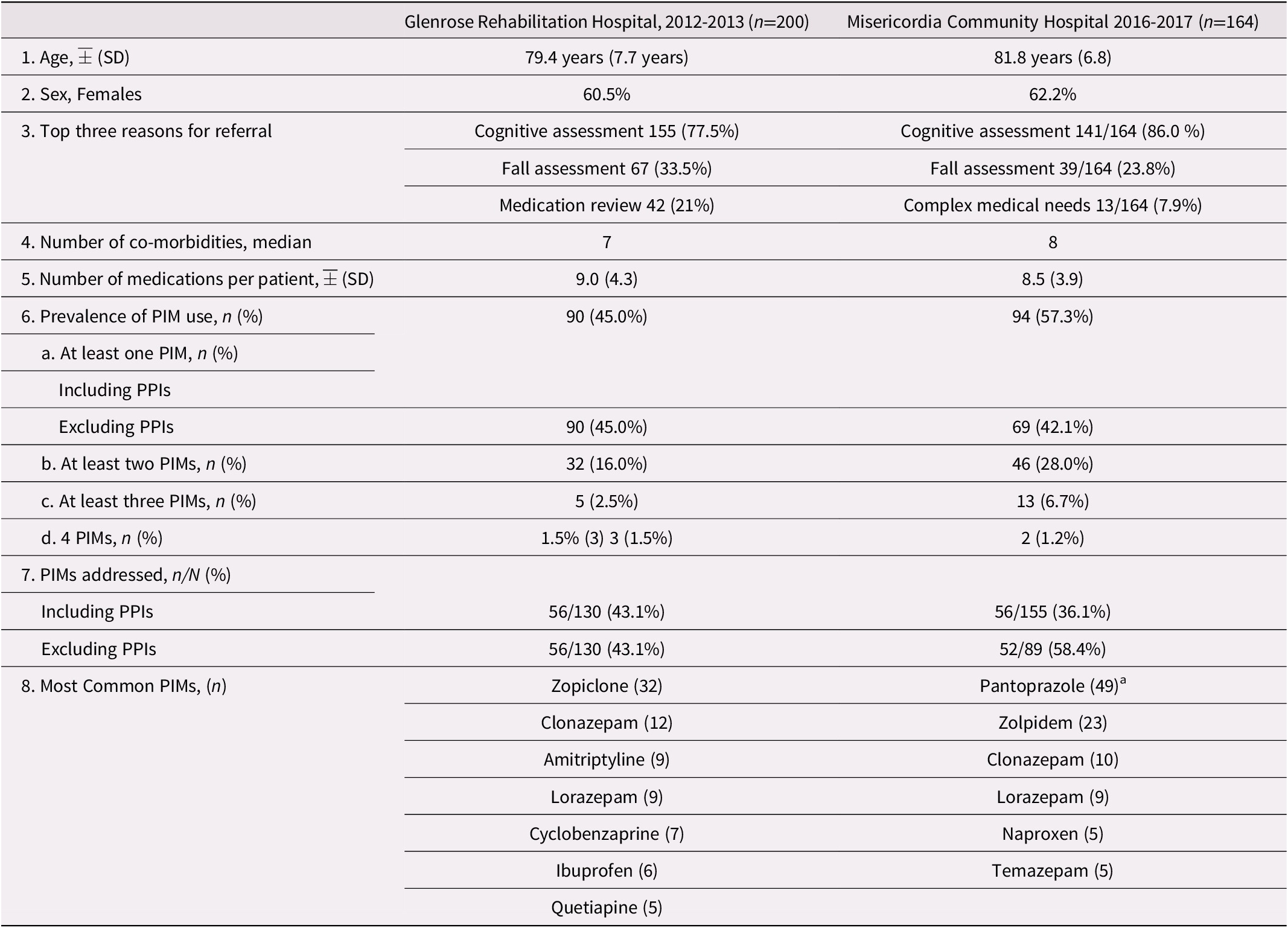
Note. aProton-pump inhibitors (PPIs), including pantoprazole, were considered as potentially inappropriate medications (PIMs) in the 2015 Beers Criteria but not in the 2012 criteria.
SD = standard deviation.
Misericordia Community Hospital
The mean age of patients was 81.8 years (SD = 6.8 years) with a predominance of females (62.2%). The median number of co-morbidities was eight. In 86.5 per cent (141/163) of cases, the reason for referral was cognitive assessment. The average number of medications per patient was 8.5 (SD = 3.9). The prevalence of PIM use was 57.3 per cent (94/164), with some patients having more than one PIM (range: 1–4 PIMs per patient). Among the 94 patients who had PIM use, a total of 155 PIMs were identified. The most frequent medications were pantoprazole, zolpidem, and clonazepam. These three drugs accounted for 64.5 per cent (82/155) of PIM use (see Table 1 and Appendix A).
Management of PIM Use after CGAs
Glenrose Rehabilitation Hospital
Of the 90 patients who had PIM use, 46.7% (42/90) had one or more PIMs stopped, tapered, decreased, and/or reviewed after the CGA. Of the 130 PIMs identified, 43.1 per cent (56/130) were ordered to be stopped, tapered, adjusted, decreased, or reviewed (see Table 2).
Table 2. Most common PIMs used and addressed
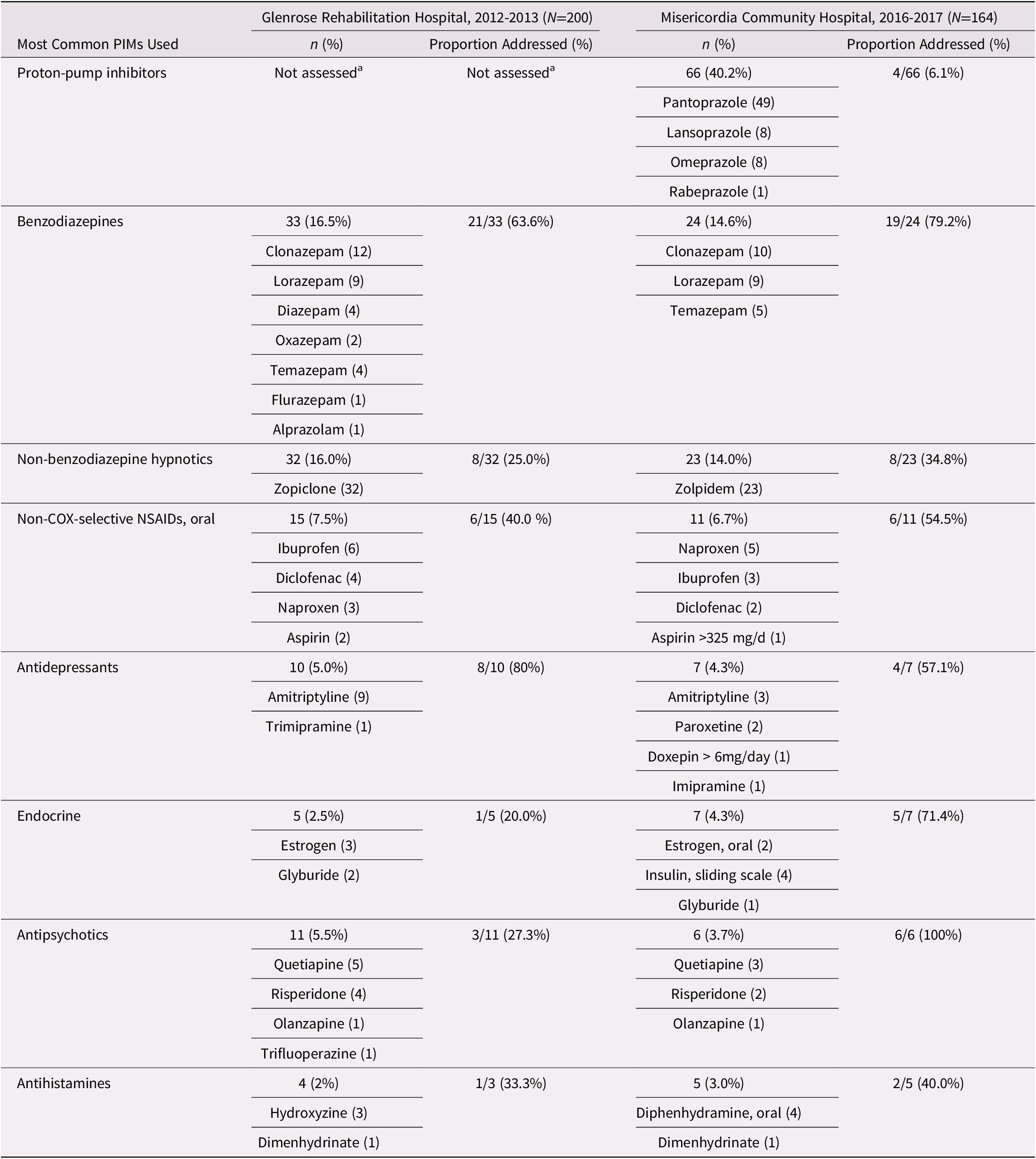
Note. aIn the 2012 Beers Criteria, proton-pump inhibitors were not classified as a PIM. In the 2015 Beers Criteria, proton-pump inhibitors were added as a PIM.
Some patients had more than one PIM.
NSAID = non-steroidal anti-inflammatory drug; PIM = potentially inappropriate medication.
Misericordia Community Hospital
Of the 94 patients who had PIM use, 47.9 per cent (45/94) had one or more PIMs stopped, tapered, decreased/increased, or reviewed after the CGA. Of the 155 PIMs identified, 36.1 per cent (56/155) were ordered to be stopped, tapered, adjusted, decreased, or reviewed. If proton-pump inhibitors were excluded from the analysis, the prevalence of PIM use decreased to 42.1 per cent (69/164), and the proportion of PIMs addressed increased to 58.4 per cent (52/89) (see Table 2).
Comparing PIM Use Between the Two Clinics
If proton-pump inhibitors are excluded, the prevalence of PIM use was slightly lower in the 2016–2017 sample than in the 2012–2013 sample: 42.1 per cent versus 45.0 per cent. However, the percentage of PIMs that were addressed, after excluding proton-pump inhibitors, is higher in the 2016–2017 sample than in the 2012–2013 sample: 58.4 per cent versus 43.1 per cent.
Discussion
Consistent with previous studies (Messerli et al., Reference Messerli, Blozik, Vriends and Hersberger2016; Zhang et al., Reference Zhang, Zhou, Pan, Li, Zhao and Zhou2017), we found a high prevalence of PIM use in both outpatient clinics over the two time periods, with the prevalence of PIM use varying from 45.0 per cent at the Glenrose outpatient clinic to 57.3 per cent at the Misericordia outpatient clinic. The increase in the prevalence of PIM use at the Misericordia site was most likely related to the change in criteria (e.g., proton-pump inhibitors being included in the 2015 PIM use criteria) between the two time periods for the chart reviews. However, if proton-pump inhibitors are excluded from our data, there were fewer PIMs being prescribed at the Misericordia outpatient clinic in 2016–2017 than in the Glenrose outpatient clinic in 2012–2013 (42.1% vs. 45.0%). Collectively, the prevalence of PIM use in older adults presenting to the two geriatric clinics highlights the need for increased education for physicians in general.
The other trend was for more PIMs being addressed at the Misericordia outpatient clinic in 2016–2017 as compared with the PIMs addressed at the Glenrose outpatient clinic in 2012–2013 (see Table 1). We postulate that with the increasing awareness of PIMs, there is less prescribing and more efforts to reduce PIM use at the later date. Our results support the Institute for Safe Medication Practices recommendation to use the Beers criteria for safer prescribing in the elderly (Institute for Safe Medication Practices Canada, n.d.).
Central nervous system (CNS) drugs and benzodiazepines have been found to be the most prescribed PIMs (Wang et al., Reference Wang, Ku, Lu, Hsu, Trezise and Wang2019). This is consistent with our findings from the Glenrose, where six of the seven most frequent medications were CNS medications (e.g., zopiclone, clonazepam, citalopram, amitriptyline, lorazepam, and olanzapine), with clonazepam and zolpidem being the top two prescribed PIMs at the Misericordia. Benzodiazepines can be difficult to deprescribe as patient buy-in is required, and there are no good alternatives for the most common reason that they are used; namely, insomnia. Success is more often achieved when patients are educated about the adverse effects of the medication that is being recommended to be deprescribed. Clinical practice guidelines and algorithms exist to mitigate this (Pottie et al., Reference Pottie, Thompson, Davies, Grenier, Sadowski and Welch2018).
In our study, we found an increased rate of PIM deprescribing if proton pump inhibitors were excluded. Many factors may play into this, including the belief that PIMs are innocuous, newer recommendations, time needed to assess if appropriate, and who recommends the discontinuation: physician versus pharmacist, despite there being decision aids for proton-pump inhibitors (Thompson et al., Reference Thompson, Farrell, Welch, Tugwell, Way and Richardson2018).
Novel results from our study also indicated that geriatrician-led CGA recommendations, if followed, would lead to decreased PIM use at two points in time (admission and discharge). This is to be expected given that geriatricians receive additional training in polypharmacy and PIMs, and this would be a good method to address PIM use given the high prevalence found on entry to the clinic. Other research has shown CGAs to significantly reduce serious adverse drug events and inappropriate medication use and polypharmacy (Mangin et al., Reference Mangin, Bahat, Golomb, Mallery, Moorhouse and Onder2018). This may have impacts on morbidity, mortality, and costs (Eamer et al., Reference Eamer, Taheri, Chen, Daviduck, Chambers and Shi2018).
It is of interest that the study by Unutmaz, Soysal, Tuven, and Isik (Reference Unutmaz, Soysal, Tuven and Isik2018) also showed that CGAs by geriatricians were helpful in decreasing PIM use, but this is the first study in North America. In addition, the use of electronic medical records (EMRs) may help with decreasing PIM use further (Kallio et al., Reference Kallio, Kumpusalo-Vauhkonen, Järvensivu, Mäntylä, Pohjanoksa-Mäntylä and Airaksinen2016; Shah, Lo, Babich, Tsao, & Bansback, Reference Shah, Lo, Babich, Tsao and Bansback2016). However, a recent study found that the configuration of alerts in an EMR was not associated with an increase in the uptake of the Beers Criteria for high hazard medications (Alagiakrishnan et al., Reference Alagiakrishnan, Ballermann, Rolfson, Mohindra, Sadowski and Ausford2019). Results are conflicting, with systematic review showing that computerized decision support tools consistently reduced the number of PIMs started and the mean number of potentially inappropriate prescriptions per patient, as well as increasing discontinuation of potentially inappropriate prescriptions and drug appropriateness. However, the results were not always significant, and further randomized controlled trials are needed (Monteiro et al., Reference Monteiro, Maricoto, Solha, Ribeiro-Vaz, Martins and Monteiro-Soares2019).
It is important to note that the AGS updated the Beers Criteria in 2019, removing ticlopidine and pentazocine because they were no longer on the United States market, and adding glimepiride, methylscopolamine, and pyrilamine (Fick et al., Reference Fick, Semla, Steinman, Beizer, Brandt and Dombrowski2019). However, this did not impact our results based on the 2012 and 2015 criteria. Studies have shown that pharmacist-led interventions can decrease PIM use (Messerli et al., Reference Messerli, Blozik, Vriends and Hersberger2016; Mogensen et al., Reference Mogensen, Thisted and Olsen2012; Nielsen et al., Reference Nielsen, Honoré, Rasmussen and Andersen2017; Patterson et al., Reference Patterson, Cadogan, Kerse, Cardwell and Bradley2014; Tommelein et al., Reference Tommelein, Mehuys, Van Tongelen, Petrovic, Somers and Colin2017). although some of these studies have been in very specific populations such as patients with cancer (Choukroun et al., Reference Choukroun, Leguelinel-Blache, Roux-Marson, Jamet, Martin-Allier and Kinowski2020). However, education targeted at physicians to decrease PIM prescribing in the first place as well as to encourage regular review may have more impact. There is also some argument that patients are more likely to comply with physician recommendations than with pharmacist recommendations (Pottie et al., Reference Pottie, Thompson, Davies, Grenier, Sadowski and Welch2018). A combined caregiver–patient-centred approach is needed to gain patient buy-in in deprescribing (Bala, Chen, & Nishtala, Reference Bala, Chen and Nishtala2019).
Research has shown that there are many factors at play with respect to PIM prescribing including: positive features of PIM, maintaining characteristics of medication intake, barriers to PIM deprescribing, system-related factors, health beliefs, and general practitioner (GP)–patient interaction (Heser et al., Reference Heser, Pohontsch, Scherer, Löffler, Luck and Riedel-Heller2018). There are also physician factors. Physicians who ranked the number of medications and benefit/risk information regarding deprescribing as more important than their peers prescribed fewer medications and PIMs after controlling for patient race and age (Ie, Felton, Springer, Wilson, & Albert, Reference Ie, Felton, Springer, Wilson and Albert2017). These should be considered when developing guidelines and educational programs aiming to reduce PIM use in the elderly. Given that decreasing PIM use is multifactorial, consideration should be given to interdisciplinary strategies to improve prescribing (Kröger et al., Reference Kröger, Wilchesky, Marcotte, Voyer, Morin and Champoux2015). To our knowledge, this is the first study showing that geriatrician-led CGA can reduce PIM use at two points in time. There are mixed results with some studies on polypharmacy and some on PIMs. A Cochrane Review in 2018 concluded it was unclear whether interventions to improve appropriate polypharmacy, such as reviews of patients’ prescriptions, resulted in clinically significant improvement despite results of a decrease in improvement in their earlier 2014 findings (Patterson et al., Reference Patterson, Cadogan, Kerse, Cardwell and Bradley2014; Rankin et al., Reference Rankin, Cadogan, Patterson, Kerse, Cardwell, Bradley, Ryan and Hughes2018). An online geriatric prescribing-education program has also proven successful in decreasing PIM use in older adults (Cullinan, Reference Cullinan2015; Cullinan, O’Mahony, & Byrne, Reference Cullinan, O’Mahony and Byrne2017). Notably, deprescribing is a complex issue requiring numerous considerations. A way forward may be the Canadian Deprescribing Network, a theoretically informed, multi-level group of organizations and individuals dedicated to advancing the deprescribing of PIMs in Canada (Tannenbaum et al., Reference Tannenbaum, Farrell, Shaw, Morgan, Trimble and Currie2017).
Limitations
A main limitation of our study was the difference in study setting, study periods, and PIMs protocol. Specifically, the two studies were distinct in that each was conducted in different settings (two different hospital settings), at two different points in time (2012–2013 and 2015–2016), with versions of PIMs that were current at that time (i.e., versions 2012 and 2015). As such, any significant differences in findings between the two reviews may be attributed to confounding from the differing time periods, hospital settings, and patient populations. Moreover, inherent in chart reviews are the limits of recorded data. This confined our results to identifying PIM use and being unaware of the benefit–harm considerations by the prescriber. Although we can infer from the recommendations that the prescriber considered the drug at hand to be a PIM, not all PIMs were addressed, and there are limits to concluding retrospectively that any given PIM is truly inappropriate. There also may have been some instances in which data were missing in past medical history (e.g., the reason for taking a proton-pump inhibitor). This may have led to an overestimation of PIMs. Also, the management of PIM use is limited to what was written in the chart, and may not reflect whether the PIM was actually stopped, tapered, or adjusted as recommended. Finally, we do not know if the new prescriptions were filled, let alone taken as directed with sustained use over time. Finally, variability in the completeness of the chart data also limited our ability to evaluate the PIMs listed in Table 3 of the Beers Criteria. As such, this variability was a potential source of underestimation of the prevalence of PIMs.
Conclusion
PIM use is highly prevalent in the community-dwelling older adult population, with many community-dwelling older adults having complex co-morbidities. CGA recommendations have lessened PIM use. This is significant, as many older adults with complex co-morbidities could benefit from geriatrician-led CGA not only in terms of the usual geriatric syndromes (e.g., cognition problems, falls) but also in reducing PIM use. An increased awareness of PIMs among physicians may further decrease PIM use.
Supplementary Materials
To view supplementary material for this article, please visit http://doi.org/10.1017/S0714980821000234.





