Introduction
Dementia is a chronic neurological condition that affects many higher cortical functions, such as memory, attention, judgement, and personality (Zivetz, Reference Zivetz1992). The estimated global prevalence of dementia in older adults is approximately 5.2 per cent, and a new case is identified every 3 seconds worldwide (Prince, Reference Prince2015). As the global population continues to age over the next 30 years, the number of persons living with dementia (PLWD) is expected to triple from 50,000,000 to 150,000,000 (Prince, Reference Prince2015).
Depression is common in PLWD, with a prevalence of between 20 and 60 per cent (Steffens et al., Reference Steffens, Otey, Alexopoulos, Butters, Cuthbert and Ganguli2006; Tsuno & Homma, Reference Tsuno and Homma2009). Up to 20 per cent of persons with Alzheimer’s disease (AD) experience a major depressive disorder (Ballard, Bannister, Solis, Oyebode, & Wilcock, Reference Ballard, Bannister, Solis, Oyebode and Wilcock1996), and in persons with vascular dementia this value rises to 50 per cent (Park et al., Reference Park, Lee, Lee, Lee, Jhoo and Youn2007). Overall, the prevalence of depression tends to be higher in persons with vascular and mixed dementias (Castilla-Puentes & Habeych, Reference Castilla-Puentes and Habeych2010; Naarding et al., Reference Naarding, de Koning, van Kooten, Dippel, Janzing and van der Mast2003; Sultzer, Levin, Mahler, High, & Cummings, Reference Sultzer, Levin, Mahler, High and Cummings1993). Depression in PLWD presents similarly to depression in persons without dementia (Engedal, Barca, Laks, & Selbaek, Reference Engedal, Barca, Laks and Selbaek2011). Depressed mood and anhedonia are present, and other manifestations can include anxiety, anger, and irritability. Sleep cycles, appetite, and energy levels can also be affected. The presence of depression in PLWD is associated with increased mortality, decreased health-related quality of life, and a reduction in the ability to conduct activities of daily living (Barbe et al., Reference Barbe, Jolly, Morrone, Wolak-Thierry, Dramé and Novella2018; Cipher & Clifford, Reference Cipher and Clifford2004; Georgakis et al., Reference Georgakis, Papadopoulos, Protogerou, Pagonari, Sarigianni and Biniaris-Georgallis2016; Petersen et al., Reference Petersen, Waldorff, Siersma, Phung, Bebe and Waldemar2017). Depression in PLWD is clinically relevant and usually requires some form of non-pharmacologic or pharmacologic intervention to minimize its impact on PLWD (Ismail & Goodarzi, Reference Ismail and Goodarzi2019).
Depression in PLWD also has a profound impact on caregivers. Many studies have found the presence of depression in PLWD is associated with increased caregiver burden (Allegri et al., Reference Allegri, Sarasola, Serrano, Taragano, Arizaga and Butman2006), caregiver depression (Cheng, Lam, & Kwok, Reference Cheng, Lam and Kwok2013), and decreased caregiver well-being (Heok & Li, Reference Heok and Li1997). In a large meta-analysis of 228 studies, the factor most strongly associated with caregiver burden and depression was behaviour problems in PLWD (Pinquart & Sörensen, Reference Pinquart and Sörensen2003). It appears that depression in PLWD has a negative impact on many aspects of caregivers’ lives. However, the extent of the impact of depression in PLWD on a broad set of caregivers is not well understood.
Given the increasing prevalence of dementia and knowing that a significant proportion of the care provided to PLWD is provided by family caregivers, family caregivers of PLWD and co-morbid depression are a group to target for more supports. However, first we need to better understand the impact of depression in PLWD on caregivers and what strategies might best support them. The current literature reviews focus on specific adverse caregiver impacts (such as burden, depression, and distress). Many of these adverse impacts do not occur in isolation, and the specific relationships between depression in PLWD and adverse caregiver impacts is unclear. There are no reviews, to our knowledge, which broadly examine the association among a wide range of adverse impacts experienced by caregivers of PLWD and comorbid depression, or that examine interventions to support these caregivers. The objectives of this scoping review are to explore the impacts that family caregivers of PLWD and co-morbid depression face, and then to determine what interventions may decrease caregiver burden and other adverse impacts.
Methods
Protocol and Research Question
Scoping reviews are “exploratory projects that systematically map the literature available on a topic, identifying key concepts, theories, sources of evidence and gaps in the research” as defined by the Canadian Institutes of Health Research (Grimshaw, Reference Grimshaw2010). The research question for this scoping review was formulated using the population, concept, and context format suggested by the Joanna Briggs Institute (Peters et al., Reference Peters, Godfrey, Khalil, McInerney, Parker and Soares2015). The population of interest was caregivers of PLWD and co-morbid depression; the concept was the impacts endured by caregivers and any potential interventions to decrease caregiver burden and other adverse impacts; the context included any study design which focused on family caregivers. Family caregivers were broadly defined to include family and friends. Caregiver adverse impacts were defined as any adverse caregiver impacts associated with depression in PLWD. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statements and guidelines were used to develop the methodology, search criteria, and data collection processes (Moher, Liberati, Tetzlaff, & Altman, Reference Moher, Liberati, Tetzlaff and Altman2009). The completed PRISMA extension for Scoping Reviews (PRISMA-ScR) Checklist can be found in Appendix A. The initial search was conducted in May 2017, and then updated in December 2019.
Search Strategy
The search strategy was developed with the help of an experienced librarian (Appendix B). Three online databases (MEDLINE®, Embase, and PsycINFO) were searched, capturing titles or abstracts containing the terms dementia, depression, words regarding aging (such as aged, senior, elder, and geriatric), and/or caregiver. Any medical subject headings (MeSH) terms that fell under these generic terms were also used in the database searches. No restrictions were placed on article location or population size. A grey literature search was also conducted in September 2018 using the Canadian Agency for Drugs and Technologies in Health (CADTH) Grey Matters tool (Appendix C), The initial search was conducted in May 2017, and the search was updated in December 2019. All references were managed using EndNote X9 and DistillerSR.
Study Selection and Eligibility Criteria
Based on the specified population, concept, and context of interest, articles were included in the scoping review if they met the following criteria: (1) focused on family caregivers, (2) involved persons with confirmed diagnoses of dementia and co-morbid depression, (3) the majority of PLWD were 60 years of age or older, (4) the article was in English, and (5) the article contained data on the relationship between depression in PLWD and caregiver impacts. Studies examining persons with young-onset dementia or Parkinson’s disease with dementia were excluded (as these have unique clinical manifestations compared with other forms of dementia in older adults), as were studies in which cognitive impairment was diagnosed by a cognitive screening tool (e.g., Mini-Mental State Examination [MMSE]) alone. Books, abstracts, and non-English articles were excluded. Studies examining interventions for caregivers and/or the PLWD and depression were included. Article inclusion was not restricted by the type of study design or year of publication.
Citation screening was conducted independently by two reviewers. Any literature which mentioned persons with co-morbid dementia and depression and information about family caregivers in the abstracts or titles were included in the full text screening stage. To increase sensitivity, any abstracts that were included by either reviewer were obtained for full text screening. During the full text screening, the articles were independently reviewed by two reviewers to determine whether inclusion criteria were met. Any disagreements were resolved through discussion.
Data Extraction and Synthesis of Results
Data extraction was done independently by one reviewer using a standardized data abstraction tool, and then reviewed with a second reviewer. Information extracted from the articles included: details of the citation (first author, publication year), the purpose of the study, study design, the time period in which the study was conducted, a description of the study population (i.e., sample size, study location), PLWD and caregiver demographic information (including age and sex), the tools (if any) used in diagnosing dementia, the tools (if any) used in diagnosing depression, the tools used to assess caregiver burden and other adverse impacts, key findings of the study, and perceived study limitations. When appropriate, the characteristics of intervention and control groups and the funding sources of intervention studies were recorded. A qualitative summary of the literature was conducted, including both numerical and narrative summaries as appropriate.
We did not appraise the quality of the studies included in our scoping review, which is consistent with the Joanna Briggs Institute Reviewer’s Manual (Peters et al., Reference Peters, Godfrey, Khalil, McInerney, Parker and Soares2015), as well as scoping reviews on clinical topics (Tricco et al., Reference Tricco, Lillie, Zarin, O’Brien, Colquhoun and Kastner2016). As the aim of our scoping review is to fully present the current state of knowledge on the various impacts that family caregivers of PLWD and co-morbid depression face, a formal appraisal of study quality was not appropriate. Detailed summaries describing the characteristics of included studies were generated.
Results
Sixteen thousand twenty-four citations were identified though the database search (Embase: 4,532; PsycINFO: 2,847; MEDLINE: 8.629) and 16 citations were identified through the grey literature search (Figure 1). After duplicates were removed, 12,835 unique citations were identified. 713 full text articles were assessed for eligibility. At the full text stage, the agreement between the two independent reviewers was 92.7 per cent. After full text screening, 91 articles were included. All the reference lists of included articles were searched, and an additional 48 articles were identified and included.
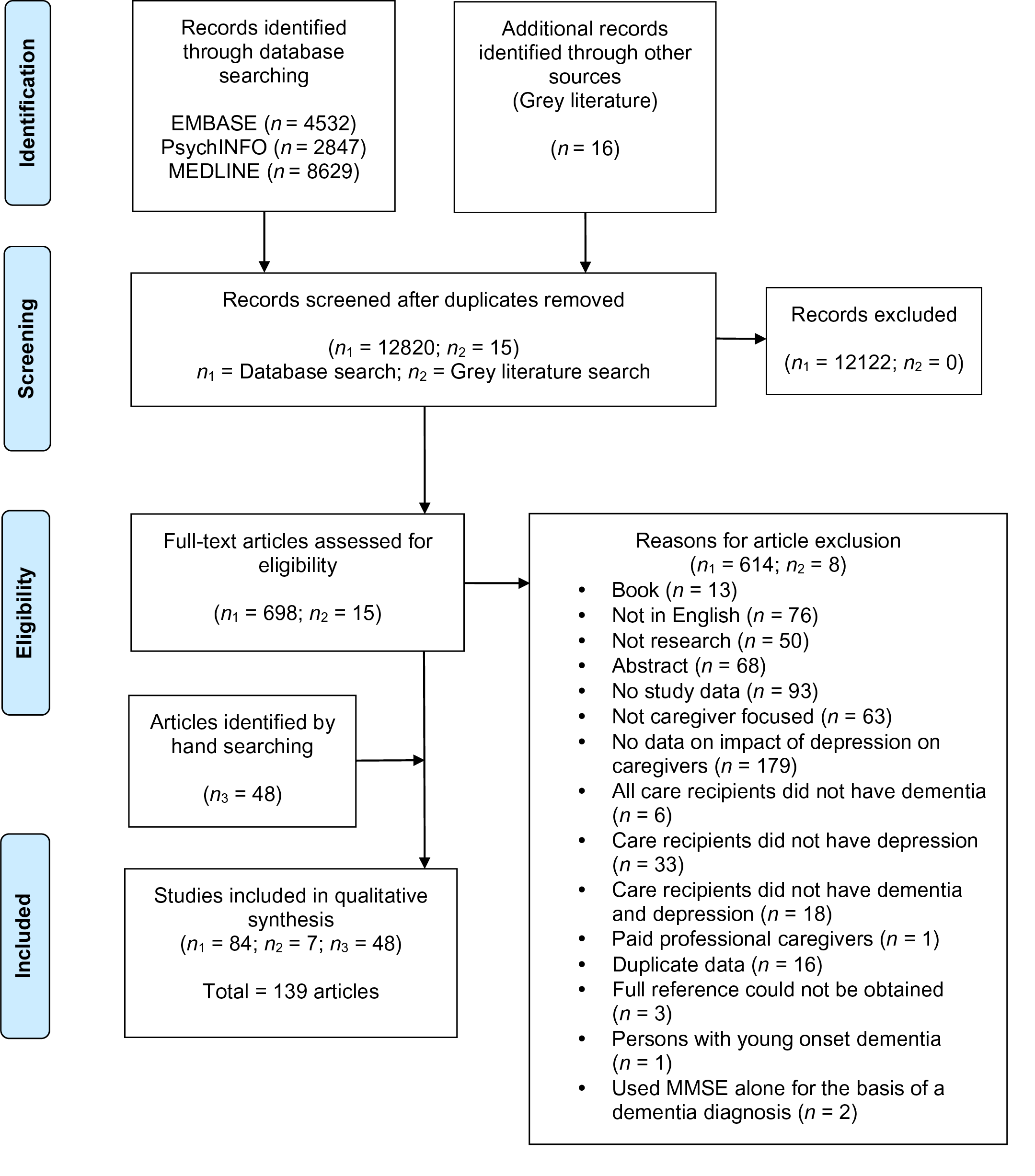
Figure 1: PRISMA flow diagram.
Ultimately, 139 articles were included in our scoping review (1–139, Appendix D). Study designs included cross-sectional (n = 106), prospective cohort (n = 14), retrospective cohort (n = 2), randomized control trial (RCT) (n = 9), quasi-experimental (n = 5), and qualitative (n = 3). The study locations were very diverse, occurring on six different continents (North America, South America, Europe, Asia, Africa, Australia). The number of care recipient/caregiver dyads ranged from 14 to 2477. The mean reported age of PLWD ranged from 60 to 85 years. The mean reported age of caregivers ranged from 42 to 78 years. The included articles reported on a variety of caregiver impacts: anxiety (n = 4), burden and strain (n = 58), burnout (n = 3), depression (n = 28), distress (n = 61), quality of life (n = 6), health and psychological well-being (n = 10), quality of care recipient/caregiver relationship (n = 1), problematic caregiving experiences (n = 1), time (n = 1), disrupted schedule (n = 1), and the economic cost of caregiving (n = 1).
Relationship between Depression in PLWD and Caregiver Impacts
One hundred twenty-four studies reported on the relationship between various caregiver impacts and depression in PLWD (Table 1, Appendix E). Most studies were cross sectional (n = 108), followed by prospective cohort (n = 14) and retrospective cohort (n = 2) designs.
Among the four studies examining caregiver anxiety, two studies reported a significant relationship (63, 80) and two reported no relationship (65, 136) (Table 1). The 52 studies examining caregiver burden and strain also reported mixed findings. Approximately 38 per cent of studies reported a significant relationship (n = 20) (5, 12, 20, 27, 32, 38, 46, 51, 56, 63, 72, 80, 87, 90, 101, 107, 114, 123, 133, 137), 37 per cent reported no relationship (n = 19) (1, 2, 10, 22, 26, 53, 54, 60, 71, 85, 93, 94, 95, 105, 111, 129, 131, 135, 136), and 11 studies (21%) had mixed findings within their own study population (21, 30, 42, 52, 61, 73, 91, 96, 112, 116, 130); 2 studies (4%) reported summary statistics only (67, 128). The results were also mixed when dementia type was considered (Appendix E). In studies examining caregiver burden related to depression in persons with AD, 11 studies reported a significant relationship (12, 20, 27, 32, 46, 61, 63, 72, 93, 101, 133), 8 reported no relationship (1, 2, 10, 22, 54, 60, 105, 136), and 4 studies had mixed results based on the analyses done (73, 112, 116, 130). In persons with frontotemporal dementia (FTD), four studies reported no relationship with caregiver burden (n = 4) (52, 54, 60, 61) or strain (n = 1) (26). The single study examining depression in persons with Lewy body dementia (DLB) found no relationship with caregiver burden (61).
Table 1: Summary of studies reporting on the burden of caregivers of persons with dementia and depression

Note. Associated article references listed are in Appendix D.
BDI = Beck Depression Inventory; BDI-II = Beck Depression Inventory, 2nd Edition; BIZA-D = German standardized burden questionnaire by Zank et al. (2006); CBI = Caregiver Burden Inventory; CES-D = Center for Epidemiologic Studies Depression Scale; CORE-10 = 10 item Clinical Outcomes in Routine Evaluation; DBS-CG = Dementia Burden Scale-Caregiver; GAD-7 = Generalised Anxiety Disorder Scale; GDS = Geriatric Depression Scale; GDS-SF-15 = Geriatric Depression Scale, 15 item short form; GHQ = General Health Questionnaire; HADS = Hospital Anxiety and Depression Scale; HRSD = Hamilton Rating Scale for Depression; MBI = Maslach Burnout Inventory; MCSI = Modified Caregiver Strain Index; NPI = Neuropsychiatric Inventory; PHQ-9 = Patient Health Questionnaire; RMBPC = Revised Memory and Behavior Problems Checklist; RSS = Relative Stress Scale; SF-12 = Short Form Mental and Physical Health Summary Scales; ZBI = Zarit Burden Inventory.
Twenty-seven studies examined caregiver depression (Table 1). The findings were mixed: approximately 50 per cent of studies found a significant relationship between depression in PLWD and caregiver depression (n = 12) (20, 29, 39, 46, 63, 72, 81, 84, 87, 92, 120, 135), whereas the others reported no relationship (n = 12) (17, 26, 33, 37, 44, 65, 71, 94, 95, 96, 129, 136) or mixed findings (n = 3) (79, 109, 133). Again, the results were still mixed by dementia type (Appendix E). Studies examining persons with AD reported a significant relationship in five studies (20, 46, 63, 72, 79), no relationship in four studies (17, 37, 65, 136), and mixed results in two studies (109, 133). The single study examining persons with FTD found no significant relationship between depression in persons with FTD and caregiver depression (26).
Fifty-three studies reported on caregiver distress (Table 1). Thirteen studies reported a significant relationship between caregiver distress and depression in PLWD (8, 18, 34, 48, 49, 50, 55, 58, 72, 77, 78, 89, 132). However, eight studies reported no relationship (13, 15, 33, 43, 93, 96, 98, 121) and four studies had mixed results (60, 81, 103, 117). The remaining studies (n = 28) reported mean or median caregiver distress scores associated with depression in PLWD (6, 7, 16, 23–25, 28, 29, 31, 36, 41, 67, 69, 70, 74, 76, 86, 97, 99, 106, 110, 115, 118, 119, 122, 124, 126, 134).
Five studies reported on caregiver quality of life (Table 1), and none found a relationship between depression in PLWD and caregiver quality of life. Nine studies reported on caregiver health and psychological well-being (Table 1). Most studies reported a significant relationship (n = 5) (14, 17, 27, 40, 57), but some did report no relationship (n = 2) (35, 68) or mixed results (n = 2) (66, 99). Studies reported that depression in PLWD was significantly associated with poorer caregiver general health (14, 17, 40), psychological well-being (57), mental health (66), and emotional health (99); feeling trapped/overwhelmed (17); and poorer physical health (99). Other studies reported no relationship between depression in PLWD and caregiver general health (35), physical health (66), and loss of physical strength (68). Overall, the relationship between depression in PLWD and caregiver health and psychological well-being is unclear.
Findings of Non-Pharmacological Intervention Studies
Seven studies examined non-pharmacological interventions (Appendix F), including the effects of cognitive behavioural therapies, education, and support provided by diverse care teams. The studies took place in Canada (n = 1) (11), the United States (n = 3) (47, 88, 100), England (n = 1) (113), and Germany (n = 2) (127, 139). The number of care recipient/caregiver dyads ranged from 50 to 389.
All studies of interventions to decrease caregiver burden reported some improvement. A quasi-experimental study of 389 care recipient/caregiver dyads reported a significant decrease in caregiver burden after the utilization of a specialized dementia care network (127). Similarly, a cluster randomized intervention trial of treatment, care, and medication management in PLWD, combined with caregiver support and education (n = 317 dyads), found that after controlling for physician, demographic data and living situation, caregivers in the intervention group showed significantly decreased burden (139). Lastly, a quasi-experimental study of 111 care recipient/caregiver dyads in which the intervention was medical management of problems in PLWD combined with caregiver education, baseline depression scores in PLWD were significantly associated with changes in caregiver burden at follow-up (11). However, change in depression scores in PLWD were not associated with change in caregiver burden scores at follow-up.
Caregiver distress appeared to lessen in some non-pharmacological intervention studies, but not in others. Caregivers (n = 129) reported significantly less distress surrounding depression in PLWD after receiving dementia services, such as assistance from crisis support coordinators and social workers, in a non-randomized concurrent control treatment outcome trial (47). Conversely, a quasi-experimental study of 127 caregivers receiving an evidence-based psychoeducational intervention showed no significant decreases in caregiver distress related to depression in PLWD (100). More surprisingly, in a pilot RCT of 60 care recipient/caregiver dyads examining a psychoeducational cognitive-behavioural intervention, caregivers in the intervention group reported being more stressed by depressive symptoms in PLWD than caregivers in the control group (88).
Findings of Pharmacological Intervention Studies
Seven studies reported findings related to pharmacological interventions (Appendix F). All the drug-based interventions aimed to improve depressive symptoms of PLWD and evaluated whether there was an impact on caregivers. Six studies were RCTs (4, 9, 62, 64, 75, 102), and one study was quasi-experimental (3). The studies took place in Spain (n =1) (3), Ukraine (n =1) (4), England (n =1) (9), Italy (n = 1) (75), and the United States (n = 3) (62, 64, 102). The number of care recipient/caregiver dyads ranged from 44 to 404. The mean reported age of PLWD ranged from 65 to 80 years. The mean reported age of caregivers ranged from 59 to 65 years. Two studies were funded by pharmaceutical companies (3, 4), four others had study authors who had worked for and/or received funding from pharmaceutical companies (9, 62, 64, 102), and one study’s funding source was unclear (75). Four studies were double blinded (4, 9, 62, 102), one study had a 1-week single blind placebo phase (64), and two studies were not blinded in any way (3, 75). The following caregiver impacts were examined: burden (n = 3) (9, 62, 102), distress (n = 5) (3, 4, 62, 64, 75), mental health (n = 1) (9), depression (n=1) (102), and quality of life (n = 1) (9). Overall, the findings were mixed. Two studies reported an improvement in caregiver impacts over the course of the study (3, 4), two studies reported no improvement (64, 102), and three studies reported mixed results (9, 62, 75).
A quasi-experimental study, examining the effects of risperidone (initial dose 1 mg/day) in 237 PLWD and comorbid depression, found mean caregiver distress scores decreased significantly over the course of the study (3). A double blinded placebo controlled RCT of 404 PLWD reported a significant decrease in caregiver distress scores with Ginkgo biloba extract Egb 761 at 240 mg/day for 24 weeks (4). A third RCT, examining the effects of sertraline (100 mg/day) in 131 persons with depression and AD, found that caregiver distress scores significantly decreased over 24 weeks in both the intervention and control groups (62). This study showed no change in caregiver burden scores in either group at the end of the study. An RCT, examining the effects of sertraline hydrochloride (mean dosage of 95 mg/day) among 44 persons with AD and depression, found no significant drug–placebo differences in total caregiver distress (64). A separate analysis of the same study population found that although caregiver burden and caregiver depression significantly decreased, these changes were not associated with intervention (102).
A non-blinded RCT examining the effects of cholinesterase inhibitor treatment (5 mg/day) plus citalopram HBr oral solution (maximum 20 mg daily) produced mixed results (75). In a correlation analysis, depression in persons with AD was correlated with caregiver stress at baseline and at the 12-month follow-up in both the intervention and control groups. However, in a univariate analysis, depression in persons with AD was only associated with caregiver stress scores in the intervention group (75). Mixed results were also found in a double blind RCT examining sertraline (150 mg/day) and mirtazapine (45 mg/day) in 326 PLWD and comorbid depression (9). At 13 weeks, caregivers of PLWD in the mirtazapine group had significantly higher quality of life scores on the mental component of the Short-Form Health Survey (SF-12) than caregivers of PLWD in the sertraline group. At 39 weeks, there were no statistically significant differences among the sertraline, mirtazapine, and placebo groups related to caregiver burden, mental health, and physical health (9).
Findings of Qualitative Studies
Three qualitative studies were included in our review (Appendix G). The studies took place in the Netherlands (45), Sweden (126), and Australia (74). All the articles aimed to describe how behavioural and psychological symptoms of dementia affected family caregivers and what strategies caregivers used to manage these symptoms. The data were obtained through semi-structured interviews (n = 2) and focus groups (n = 1). The number of care recipient/caregiver dyads ranged from 14 to 32.
In all three studies, caregivers reported distress related to depression in PLWD. One study involving 32 caregivers described what particular aspects caregivers found stressful, and the overall finding were: (1) the continual switching of the PLWD’s behaviour, (2) continually keeping the person with dementia occupied and diverted, (3) the fact that others see a different side to their relative, and (4) knowing what to do in theory but being unable to put it into practice (45). The main theme of another study examining 14 spousal caregivers was “living on the edge, lacking social support and time for self.” The themes that emerged in this study were particularly alarming, as many caregivers reported dealing with unpredictable hostile situations, feeling trapped, social isolation, and being vulnerable to domestic violence (126).
Discussion
In Poulshock and Deimling’s model of caregiver strain (Poulshock & Deimling, Reference Poulshock and Deimling1984), dementia leads to problematic behavioural and psychological symptoms (BPSD), which places burden on caregivers and ultimately leads to physical, psychological, social, and financial difficulties for caregivers. The current literature has clearly established the strong relationship between caregiver burden and the presence of dementia. This scoping review examined whether the addition of depression in PLWD was associated with adverse caregiver impacts/outcomes. This scoping review revealed the complexity of the relationship between comorbid depression in PLWD and various adverse caregiver impacts/outcomes Specifically, the impact on caregiver burden, strain, and burnout is unclear. Approximately 50 per cent of all studies examining these caregiver variables reported statistically significant relationships, while the other 50 per cent reported no relationship. These mixed findings persisted even when the findings were broken down by dementia type and when only cohort studies were considered. Similarly, the results surrounding caregiver depression and anxiety were mixed. Again, approximately 50 per cent of studies examining these topics found a significant relationship, whereas the other half found no significant relationship. The type of dementia and the study design were not contributing factors. The relationship between depression with PLWD and caregiver health and psychological well-being is also unclear.
However, caregiver distress does appear to be related to depression in PLWD, with a large number of studies supporting this relationship. This suggests that although depression in PLWD is distressing to those caring for them, it does not seem to consistently add to the burden experienced by caregivers or consistently impact their own mental health. Caregiver distress as a concept is more closely related to caregiver burden than caregiver depression. Many of the studies which examined caregiver depression used validated depression scales such as the Beck Depression Inventory and the Geriatric Depression Scale, whereas most studies examining caregiver distress used the Neuropsychiatric Inventory-Caregiver Distress Scale. Similarly, caregiver quality of life does not appear to be impacted. Caregiver quality of life is a variable that encompasses many components, such as physical health, mental health, living situation, and financial circumstances (Logsdon, Gibbons, McCurry, & Teri, Reference Logsdon, Gibbons, McCurry and Teri1999). Therefore, it is not surprising that caregiver quality of life is not significantly associated with the presence of depression in PLWD.
These results overall are somewhat surprising, given that it is generally thought that depression in PLWD is burdensome on caregivers (Allegri et al., Reference Allegri, Sarasola, Serrano, Taragano, Arizaga and Butman2006; Cheng et al., Reference Cheng, Lam and Kwok2013; Lethin et al., Reference Lethin, Leino-Kilpi, Bleijlevens, Stephan, Martin and Nilsson2020). To reiterate, the research on caregiver burden among caregivers of PLWD is very well established. There is a definite relationship between the presence of dementia and caregiver burden. What this scoping review assessed was the relationship between the presence of depression in PLWD and adverse caregiver impacts (one of which was caregiver burden). This scoping review suggests that the presence of depression in PLWD may be associated with additional burden, distress, and other adverse impacts for some caregivers but not for others. This could be because depression can be missed in PLWD, caregivers may not feel comfortable discussing their own adverse impacts, or some caregivers may not find the depressive symptoms distressing.
The relationship between PLWD and their family caregivers seems to be complex, in that depression is probably just one factor contributing to caregiver impacts. Pearlin and colleagues proposed a model in which many factors play a role in caregiver impacts (Pearlin, Mullan, Semple, & Skaff, Reference Pearlin, Mullan, Semple and Skaff1990). These include background and context, primary stressors (i.e., BPSD, PLWD limited ability to perform activities of daily living), secondary role strains (i.e., family conflict, economic challenges), and secondary intrapsychic strains (i.e., caregiver self-esteem, competence, mastery). Additionally, this model suggests that there are variables which mediate all these relationships, such as the availability of social supports. It has also been suggested that depression in caregivers is related to caregiver burden (Epstein-Lubow, Davis, Miller, & Tremont, Reference Epstein-Lubow, Davis, Miller and Tremont2008). Others have hypothesized that caregiver personality styles and coping strategies mediate the relationship between BPSD (like depression in PLWD) and caregiver burden (Baharudin, Din, Subramaniam, & Razali, Reference Baharudin, Din, Subramaniam and Razali2019). Recently, Ying and colleagues developed a framework to assist in the prevention of depression in caregivers of PLWD (Ying, Yap, Gandhi, & Liew, Reference Ying, Yap, Gandhi and Liew2018). In this framework, psychical care demands, BPSD, lower caregiving competency, and loss and grief lead to perceived stress or difficulty in coping, which lead to caregiver depression. This framework targeted interventions to address BPSD in PLWD, caregiver feelings of loss and grief, physical care demands, and caregiving competency, with the goal of helping to prevent caregiver depression.
Most studies did not control for additional caregiver and care recipient factors when examining the relationship between depression in PLWD and caregiver impacts. Because of the inter-relatedness of many caregiver variables and the impact of other care recipient challenges, it is impossible to describe the relationship between depression in PLWD and caregiver impacts without controlling for potential confounding factors. Indeed, when studies included in this scoping review controlled for confounding factors, they tended to report no relationship between depression in PLWD and caregiver impacts. For example, in one study in which a multiple regression analysis controlled for problem-focused coping strategies, coping strategies using self-blame, and the memory and depression subscale of the Revised Memory and Behavior Problems Checklist (RMBPC), the depression subscale of the RMBPC was not correlated with caregiver burden (Huang et al., Reference Huang, Huang, Su, Hou, Chen and Yeh2015). Similarly, in another study which employed a multivariate adjusted multiple regression model which adjusted for demographics, living situation, financial status, physical function, cognitive status, mood, nutritional status, frailty, polypharmacy, and the dementia behavioural disturbance scale scores, depression in persons with dementia was not significantly associated with caregiver burden (Sugimoto et al., Reference Sugimoto, Ono, Kimura, Saji, Niida and Toba2018).
We examined both pharmacological and non-pharmacological interventions to determine whether caregiver issues related to depression in PLWD improved. The non-pharmacological interventions tended to target caregivers and were broader in their target of improving caregiver impacts. Therefore, the non-pharmacological interventions examined in this article may also be beneficial to caregivers of PLWD who do not have depression. Conversely, the pharmacological interventions included in this scoping review directly target depressive symptoms in PLWD and only indirectly affect caregiver variables. Therefore, these pharmacological interventions are only appropriate for PLWD who have depression.
Non-pharmacological interventions such as cognitive behavioural therapies, education, and support provided by diverse care teams showed reduced caregiver burden related to depression in persons with dementia, but their impact on caregiver distress was mixed. Surprisingly, not all the caregiver outcomes reported were positive. One pilot randomized control trial examining a psychoeducational cognitive-behavioural intervention found that caregivers in the intervention group reported being more stressed by depressive symptoms in PLWD (88). This may have occurred because caregivers in the intervention group became trained to better identify depression in PLWD and were more aware of the negative outcomes associated with depression, which caused increased stress. Based on these findings, non-pharmacological studies may have a specific role in helping to reduce caregiver burden related to depression in PLWD. The literature on non-pharmacological interventions to treat BPSD is in line with the findings of our scoping review. A recent systematic review examining the impact of non-pharmacological interventions on BPSD found that occupational activity interventions may reduce depression in PLWD (de Oliveira et al., Reference de Oliveira, Radanovic, de Mello, Buchain, Vizzotto and Celestino2015). Another systematic review on non-pharmacological interventions for BPSD in nursing homes also found that interventions such as light therapy and multisensory therapy significantly reduced depression in PLWD (Wang, Albayrak, & van der Cammen, Reference Wang, Albayrak and van der Cammen2019). Conversely, pharmacological interventions mostly explored the impact on caregiver distress. Overall, there were few studies, and many were funded by pharmaceutical companies. Therefore, non-pharmacological interventions should be attempted first.
Clinicians should be cautious when prescribing antidepressants to PLWD. A recent Cochrane systematic review on the use of antidepressants for treating depression in PLWD found variable evidence of the efficacy of antidepressants in this population, especially after 12 weeks of use (Dudas, Malouf, McCleery, & Dening, Reference Dudas, Malouf, McCleery and Dening2018). Additionally, PLWD who were assigned to intervention groups were more likely to drop out, citing having one or more unwanted side effects associated with antidepressant use (Dudas et al., Reference Dudas, Malouf, McCleery and Dening2018). Combined with the fact that they do not appear to clearly improve caregiver distress or burden, antidepressants do not have a clear role in managing depression in PLWD.
It is important to remember that statistical significance is not equivalent to clinical significance. The relationship between two variables can be statistically significant but not clinically significant, and vice versa. Although the relationship between depression in PLWD and various caregivers’ impacts may not have been statistically significant in many studies, this does not mean that depression in PLWD does not have clinically significant impacts on caregivers and their lives. We recommend that future studies be longitudinal in nature and control more for potential confounders, particularly caregiver-related variables, to determine the exact nature of the impact of depression in PLWD on caregiver impacts. Further, future work should examine potential factors that mediate the relationship between depression in PLWD and caregiver impacts.
This scoping review presents international findings with very different policy and program contexts. Although the findings related to the various adverse caregiver impacts are mixed, there is evidence to suggest that non-pharmacologic interventions can improve caregiver burden related to depression in PLWD. Many of these interventions benefit all caregivers of PLWD, not just those caring for PLWD who have comorbid depression. Therefore, early referral and recommendation to non-pharmacologic interventions, even before the manifestation of depressive symptoms, could help support caregivers of PLWD with comorbid depression, and avoid many of the challenges and side effects associated with the prescription of antidepressants to PLWD.
Limitations
Our comprehensive scoping review has some limitations. First, as we only included English language articles, relevant articles in other languages were missed. This limitation is somewhat mitigated by the extensiveness of our review and that included studies came from a wide variety of countries on six different continents. Second, as many included studies examining how depression in PLWD was related to caregiver impacts were cross sectional, conclusions regarding causation cannot be clearly drawn. Third, the specific findings of this scoping review are only generalizable to older adults over the age of 60 living with dementia.
Conclusion
Depression in PLWD appears to be associated with caregiver distress. However, the impact of depression in PLWD on various other caregiver adverse impacts is more complicated. There is some evidence to suggest that non-pharmacological interventions can reduce caregiver burden related to depression in PLWD. The evidence for pharmacological interventions is less clear. More large longitudinal studies, which control for a diverse set of caregiver and care recipient confounding factors, will allow us to better understand the relationship between depression in PLWD and caregiver impacts.
Funding
A. Subota receives graduate funding from the Canadian Institutes of Health Research, the Brenda Stafford Foundation Chair in Geriatric Medicine, and the Cumming School of Medicine. N. Spotswood received funding as an Alberta Health Services Seniors Health Strategic Clinical Network Summer Research student. J. Holroyd-Leduc is funded as the University of Calgary Brenda Strafford Foundation Chair in Geriatric Medicine. This work was supported by the Seniors Health Strategic Clinical Network 2018 Summer Studentship and by the University of Calgary Brenda Strafford Foundation Chair in Geriatric Medicine. The funders played no role in the scoping review.
Supplementary Materials
To view supplementary material for this article, please visit http://doi.org/10.1017/S071498082100060X.