Introduction
Subcortical ischemic vascular cognitive impairment (SIVCI) is the second most common cause of cognitive impairment and dementia (Prins & Scheltens, Reference Prins and Scheltens2015). It is associated with chronic ischemia resulting in white matter hyperintensities (WMHs) (Prins & Scheltens, Reference Prins and Scheltens2015). Epidemiological data consistently suggest that WMHs are progressive in nature (Gouw et al., Reference Gouw, van der Flier, Fazekas, van Straaten, Pantoni and Poggesi2008; Prins & Scheltens, Reference Prins and Scheltens2015) and that females show greater WMH burden, as well as greater rate of progression, than males (Sachdev, Parslow, Wen, Anstey, & Easteal, Reference Sachdev, Parslow, Wen, Anstey and Easteal2009). Previous research indicates that aerobic training (AT) may be a promising strategy to reduce vascular risk factors (Lakka & Laaksonen, Reference Lakka and Laaksonen2007), and thus has the potential to mitigate WMH progression in people with SIVCI. Hence, the goal of this exploratory analysis of data acquired from a randomized controlled trial (RCT) was to investigate the effect of 6 month, thrice-weekly, AT on WMH progression, and to determine whether these changes are sex dependent. The examination of sex differences in health research is a timely endeavor, as this is currently lacking within the context of Canadian RCTs (Welch et al., Reference Welch, Doull, Yoganathan, Jull, Boscoe and Coen2017).
Methods
Ethical approval was obtained from the Vancouver Coastal Health Research Institute (V07-01160) and the University of British Columbia’s Clinical Research Ethics Board (H07-01160). All subjects gave written informed consent in accordance with the Declaration of Helsinki.
Study Design
This is a secondary and exploratory analysis of a proof-of-concept RCT designed to assess the effect of a 6 month, thrice-weekly, progressive AT program on cognitive function in older adults with mild SIVCI (Liu-Ambrose et al., Reference Liu-Ambrose, Best, Davis, Eng, Lee and Jacova2016). Eligible participants were randomized into either a 6 month AT or a usual care (control [CON]) group. This study used measurements taken at baseline and at trial completion (6 months post-randomization). A subset of participants volunteered and were eligible for magnetic resonance imaging (MRI), which was completed at baseline and trial completion (6 months). These scans were used for WMH volume quantification. The design and primary results of the Promotion of the Mind Through Exercise (PROMoTE) study have been published previously (Liu-Ambrose et al., Reference Liu-Ambrose, Eng, Boyd, Jacova, Davis and Bryan2010, Reference Liu-Ambrose, Best, Davis, Eng, Lee and Jacova2016).
Participants
Participants were recruited from the University of British Columbia Hospital Clinic for Alzheimer’s Disease (AD) and Related Disorders, the Vancouver General Hospital Stroke Prevention Clinic, and specialized geriatric clinics in Metro Vancouver, British Columbia. Clinical diagnosis of SIVCI was confirmed in each participant by a neurologist, based on the presence of cerebral small vessel disease and mild cognitive impairment (Erkinjuntti, Reference Erkinjuntti2002). Clinical computerized tomography (CT) or MRI scans were used to ascertain the presence of cerebral small vessel disease, defined as the presence of periventricular and deep white matter lesions and the absence of cortical-subcortical non-lacunar territorial infarcts and watershed infarcts, hemorrhages indicating large vessel disease, signs of normal-pressure hydrocephalus, or other specific signs of white matter lesions (i.e., multiple sclerosis, leukodystrophies, sarcoidosis, or brain irradiation). Mild cognitive impairment was defined as a Montreal Cognitive Assessment (MOCA) (Nasreddine et al., Reference Nasreddine, Phillips, Bédirian, Charbonneau, Whitehead and Collin2005) score < 26/30. Diagnosis of SIVCI also required evidence of progressive cognitive decline (compared with previous level of cognitive function) as confirmed through medical records or caregiver/family member interviews. Overall, participants were community dwelling and living independently with minimal assistance from family or caregiver.
Inclusion Criteria
Both inclusion and exclusion criteria have been published previously (Liu-Ambrose et al., Reference Liu-Ambrose, Eng, Boyd, Jacova, Davis and Bryan2010). Briefly, individuals were eligible for study entry if they met the following criteria: (1) being ≥ 55 years of age, (2) MOCA score < 26/30 at screening (Nasreddine et al., Reference Nasreddine, Phillips, Bédirian, Charbonneau, Whitehead and Collin2005) , (3) Mini-Mental State Examination (MMSE) score ≥ 20 at screening (Folstein, Folstein, & McHugh, Reference Folstein, Folstein and McHugh1975), (4) if on a cognitive medication (e.g., donepezil, galantamine, rivastigmine, memantine,), remaining on a fixed dosage during the study period, and (5) providing written informed consent.
Exclusion Criteria
Exclusion criteria included: (1) being diagnosed with dementia of any type (e.g., Alzheimer’s disease, vascular dementia, dementia with Lewy bodies, frontal-temporal dementia) or other neurological conditions (e.g., multiple sclerosis, Parkinson’s disease); (2) taking medications that may negatively affect cognitive function, such as anticholinergics, including agents with pronounced anticholinergic properties (e.g., amitriptyline), major tranquilizers (typical and atypical antipsychotics), and anticonvulsants (e.g., gabapentin, valproic acid); and (3) participation in a clinical drug trial concurrent to this study.
Descriptive Variables
At baseline, information regarding age, sex, body mass index (BMI = weight in kg/height in m2), waist-to-hip ratio (waist circumference/hip circumference), and global cognition (MMSE and MOCA) were collected. We also acquired information on co-morbidities using the Functional Comorbidity Index, which estimates the degree of co-morbidity associated with physical functioning (Groll, To, Bombardier, & Wright, Reference Groll, To, Bombardier and Wright2005). Depression was assessed using the 15 item Geriatric Depression Scale (Yesavage, Reference Yesavage1988).
Covariates: Apolipoprotein E (ApoE) ε4 Genotype
ApoE genotype was determined using TaqMan® Assay Systems for the single nucleotide polymorphisms: 219G/T. DNA was extracted from whole blood using an automated DNA extraction machine (AutogenflexStar, Autogen Inc, Hollisten, MA). Because the ApoE ε4 genotype is relatively rare, the ApoE ε4 genotype odds ratios were collapsed into two main categories: those with at least one ε4 allele or those with no ε4 allele (Hsiung, Sadovnick, & Feldman, Reference Hsiung, Sadovnick and Feldman2004).
Dependent Variables
Change in WMH volume
Scanning protocol
Structural MRI data was acquired at baseline and trial completion on a Philips 3T Achieva MRI scanner (Philips Medical Systems, Best, The Netherlands) at the UBC MRI Research Centre. An axial T2-weighted scan and proton density (PD)-weighted scan were acquired for each subject. The repetition time (TR) and echo time (TE) for the T2-weighted images were TR = 5431 and TE = 90 ms and for the PD-weighted images were TR = 2000 ms and TE = 8 ms. Dimensions for the T2- and PD-weighted scans were 256 × 256 × 60 voxels with a voxel size of 0.937 × 0.937 × 3.000 mm.
Image analysis
All WMH volume was computed using MRI scans. Magnetic resonance images were preprocessed using standard and publicly available neuroimaging tools that included: (1) MR intensity inhomogeneity correction using a multiscale version of the nonparametric non-uniform intensity normalization method (N3) (Sled, Zijdenbos, & Evans, Reference Sled, Zijdenbos and Evans1998), (2) application of a structure-preserving noise-removal filter (Smallest Univalue Segment Assimilating Nucleus [SUSAN]) (Smith & Brady, Reference Smith and Brady1997), and (3) removal of all non-brain tissues using the brain extraction tool (BET) (Smith, Reference Smith2002).
WMHs were then identified and digitally marked by a radiologist with experience in WMH identification. The radiologist was blinded to all participant information, including treatment assignment. Baseline and 6 month scans were co-registered and reviewed together to ensure consistency of identification of small lesions across time. The radiologist used the following guidelines in the seeding procedure, which was designed to be efficient and intuitive: (1) mark all distinct WMHs regardless of size, (2) place more than one point on a lesion if the additional points would help define the extent of the lesion, and (3) place at least one point near the center of each lesion (McAusland, Tam, Wong, Riddehough, & Li, Reference McAusland, Tam, Wong, Riddehough and Li2010).
WMHs were segmented by a method that automatically computed the extent of each marked lesion (McAusland et al., Reference McAusland, Tam, Wong, Riddehough and Li2010). This segmentation method has been validated in large data sets with a wide range of lesion loads. It was found to be highly accurate compared with radiologist segmentations and also robust to variations in the placement of seed points (McAusland et al., Reference McAusland, Tam, Wong, Riddehough and Li2010). Full details on the point placement procedure and subsequent automatic segmentation are described in previous work (McAusland et al., Reference McAusland, Tam, Wong, Riddehough and Li2010). Briefly, the seed points were processed by a customized Parzen windows classifier (Parzen, Reference Parzen1962) to estimate the intensity distribution of the lesions. The algorithm included heuristics to optimize the accuracy of the estimated distributions by dynamically adjusting the position and the number of seed points used for the Parzen window computation, as well as a spatial method that approximated visual shape partitioning to identify areas that were likely to be false positives (McAusland et al., Reference McAusland, Tam, Wong, Riddehough and Li2010). The lesion masks were then used to quantify WMH volumes in mm3. All lesion masks were reviewed by a trained research assistant to ensure accuracy.
Change in general functional cardiovascular capacity
General functional cardiovascular capacity was assessed using the 6-Minute Walk Test (6MWT). Participants were instructed to walk as quickly as possible for 6 minutes, and the distance walked in meters was recorded. Performance on this test has been used as an indicator of cardiovascular capacity. It is correlated with peak oxygen uptake and is used in RCTs as a fitness outcome measure (Kervio, Carre, & Ville, Reference Kervio, Carre and Ville2003).
Randomization and Allocation Concealment
Participants were randomly assigned (1:1) to either a 6 month AT or CON group. The randomization sequence was generated by a web-based randomization service, at http://www.randomization.net.
Experimental Group: AT Group
Certified exercise instructors led all AT classes. Classes occurred three times a week, and each class was 60 minutes long and consisted of 40 minutes of walking with a 10 minute warm-up and cool down session. Participants walked along a predetermined outdoor route. Over the 6 month intervention period, we progressed the intensity of the AT program and monitored participants’ progression with the following techniques: (1) Participants wore a heart rate monitor and were initially asked to work at approximately 40 per cent of their age-specific target heart rate (i.e., heart rate reserve). Over the first 12 weeks, participants were asked to gradually increase the intensity to 60–70 per cent of heart rate reserve, with the target being 65 per cent. Once the target was achieved, it was sustained by the participant for the remainder of the intervention period. (2) We subjectively monitored the intensity of each workout using the Borg’s Rating of Perceived Exertion with a target of 14–15. (3) We used a “talk” test in which participants were asked to initially walk at a pace at which they could converse comfortably without effort and gradually progress to a pace at which conversation required some effort.
CON Group: Usual Care Plus Education Group
Participants in the CON group met monthly in a group setting to receive educational material about SIVCI and a healthy diet. This information was also offered to participants in the AT group. In addition, research staff phoned the CON participants on a monthly basis to maintain contact and to acquire research data.
Compliance and Adverse Events
Compliance was calculated based on the percentage of total classes attended. To monitor adverse effects, participants were questioned about the presence of any discomforts (i.e., musculoskeletal pain) at each session. During AT, instructors also monitored participants for symptoms of angina pectoris and shortness of breath.
Statistical Analysis
All statistical analyses were performed using Statistical Package for the Social Sciences 24.0. Data were checked to ensure that the assumptions of analysis of covariance were met. Plots of standardized residuals indicated that the data were normally distributed and homoscedastic.
To assess change in functional cardiovascular capacity, a 2 × 2 analysis of covariance was conducted to determine the effect of group (AT vs. CON), sex, and group × sex interaction. The dependent variable was change in 6MWT (trial completion minus baseline values; higher values indicate improved performance). We controlled for baseline 6MWT performance, age, and BMI. If a significant interaction was identified, planned pairwise comparisons (with Bonferroni corrected p values) were done to examine sex differences within and between groups.
To determine the effect of AT and sex on change in WMH volume, we conducted a 2 × 2 analysis of covariance to determine the effect of group (AT vs. CON), sex, and group × sex interaction. The dependent variable was change in WMH volume (trial completion minus baseline WMH volume; higher values indicate increased WMH volume at trial completion). We controlled for baseline WMH volume, age, and ApoE ε4 status. If a significant interaction was identified, planned pairwise comparisons (with Bonferroni corrected p values) were done to examine sex differences within and between groups.
Results
Participants
Seventy eligible participants consented and were randomized in the PROMoTE study (Liu-Ambrose et al., Reference Liu-Ambrose, Best, Davis, Eng, Lee and Jacova2016). Of the 70 participants, 29 completed MRI scanning at baseline and trial completion; 16 were randomized to the AT group and 13 were randomized to the CON group.
Table 1 reports baseline descriptive characteristics of the 29 participants. Compared with the remaining participants in the parent study, this subset had a higher mean MOCA score (mean difference [MD] = 1.86, p = .05). No other differences in descriptive characteristics were observed, including age (p > .05) and MMSE score (p > .05). Furthermore, independent samples t-test reported no significant baseline differences in WMH volume (p > .05) or 6MWT (p > .05) between AT and CON females or males.
Table 1: Descriptive characteristics
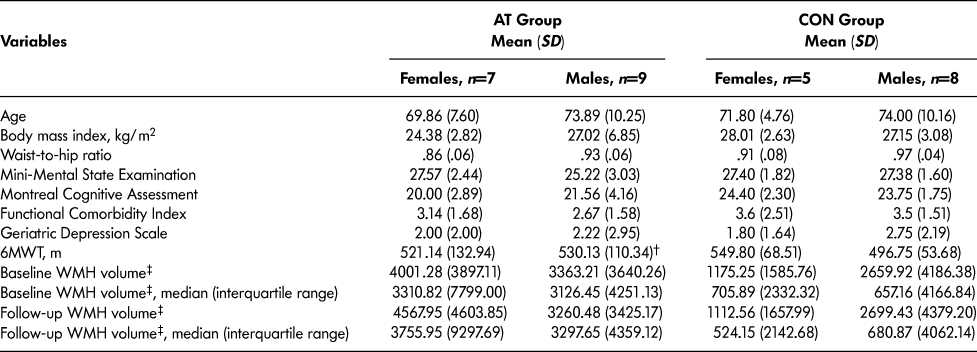
Note. 6MWT = 6-Minute Walk Test, WMH = White matter hyperintensity, †n = 8, ‡WMH volume measured in mm3.
Compliance and Adverse Events
Within this subset, the average exercise compliance in the AT group was 72 per cent. One study-related adverse event was reported in the AT group and none were reported in the CON group. The AT-related adverse event was a non-syncopal fall. The participant who experienced this continued with the study and completed all assessments.
Change in General Functional Cardiovascular Capacity
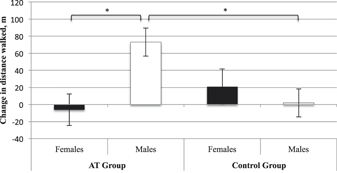
Accounting for baseline 6MWT, age, and BMI, we found no main effect of group (p = .24) or sex (p = .11). However, there was a significant sex × group interaction (F [1, 21] = 6.90, p = .02, ηp2 = .25). AT males displayed improved functional cardiovascular capacity compared with CON males (MD = 71.18 m, standard error [SE] = 23.20 m, p Bonferroni = .01). There was no significant difference between females in the AT and CON groups (p Bonferroni = .36). Within the AT group, males displayed greater improvement in functional cardiovascular capacity than females (MD = 79.23 m, SE = 25.34 m, p Bonferroni = .01). Within the CON group, males and females did not differ (pBonferroni = 0.49). For estimated marginal means please refer to Table 2 and Figure 1.
Table 2: Estimated adjusted means (standard error) for change in 6MWT performance and WMH volume from baseline to trial completion (6 months)

Note. *Change in 6-Minute Walk Test (6MWT) performance was adjusted for baseline 6MWT, age, and body mass index. There was a significant sex × group interaction, p = .02.
† n = 8
‡ Change in white matter hyperintensity (WMH) volume was adjusted for baseline WMH volume, age, and ApoE ε4 status. There was a significant sex × group interaction, p = .03.
Change values were calculated as 6 months minus baseline. Higher values indicate improved performance on 6MWT and increased WMH volume.
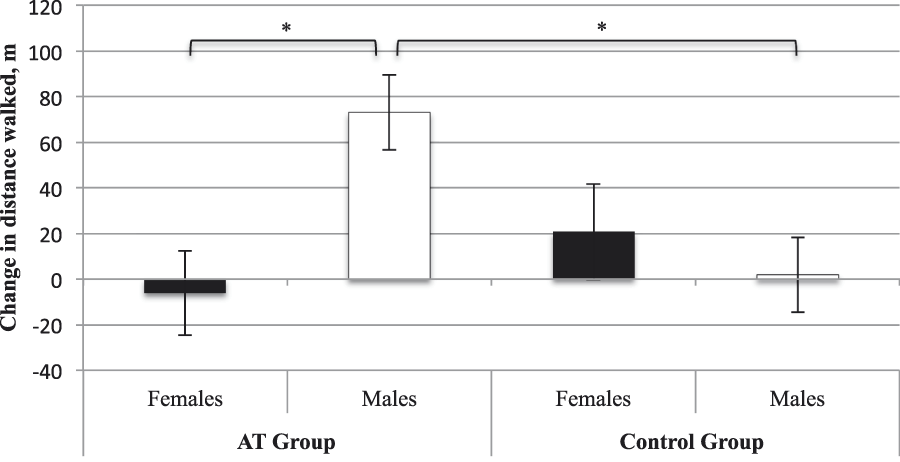
Figure 1: Estimated adjusted means (standard error) for change in 6-Minute Walk Test (6MWT) performance from baseline to trial completion. Change values were calculated as 6 months minus baseline. Higher values indicate improved 6MWT performance. * Significantly different at p ≤ .05
Interaction Effect between AT and Sex on WMH Volume
Accounting for baseline WMH volume, age, and ApoE ε4 status, we found no significant main effect of group (p = .33) or sex (p = .09). However, there was a significant sex × group interaction (F [1, 22] = 5.43, p = .03, ηp2 = .20). Over the 6 month study, AT females, who had a greater average baseline WMH volume than CON females, demonstrated greater WMH progression than CON females (MD = 491.92 mm3, SE = 235.08 mm3, p Bonferroni = .05). Among males, there were no significant between-group differences in WMH volume change (p Bonferroni = .31). Within the AT group, males demonstrated significantly less WMH progression than females at 6 months (MD = 595.36 mm3, SE = 194.30mm3, p Bonferroni = 0.01). Within the CON group, there were no significant sex differences in WMH volume change (p Bonferroni = .69). For estimated marginal means please refer to Table 2 and Figure 2.

Figure 2: Estimated adjusted means (standard error) for change in white matter hyperintensity (WMH) volume from baseline to trial completion. Change values were calculated as 6 months minus baseline. Higher values indicate increased WMH volume. * Significantly different at p ≤ .05
Discussion
We found that 6 months of moderate-intense AT did not have a significant effect on WMH progression among older men and women with mild SIVCI. However, our results suggest that the effects of moderate-intense AT on WMH progression may vary by sex. Among males, there were no group differences in WMH progression. Females in the AT group, who demonstrated the greatest burden of WMH volume at baseline, continued to demonstrate significantly greater WMH progression compared with CON females at trial completion. We note that these results must be interpreted within the context of white matter pathology. The Personality and Total Health (PATH) Through Life Project (Sachdev et al., Reference Sachdev, Parslow, Wen, Anstey and Easteal2009) and the Rotterdam Scan Study (de Leeuw et al., Reference de Leeuw, de Groot, Achten, Oudkerk, Ramos and Heijboer2001) reported greater WMH burden in older women than in older men, in both periventricular and deep white matter regions. Furthermore, longitudinal data from the PROspective Study of Pravastatin in the Elderly at Risk (PROSPER) study found that women had approximately twice as much deep WMHs as men over a 3 year period (van den Heuvel et al., Reference van den Heuvel, Admiraal-Behloul, ten Dam, Olofsen, Bollen and Murray2004). Critically, several studies have indicated that the strongest predictor of WMH progression is baseline WMH volume, whereby greater WMH burden at baseline predicts greater WMH progression (Gouw et al., Reference Gouw, van der Flier, Fazekas, van Straaten, Pantoni and Poggesi2008; Sachdev et al., Reference Sachdev, Parslow, Wen, Anstey and Easteal2009; Schmidt, Fazekas, Kapeller, Schmidt, & Hartung, Reference Schmidt, Fazekas, Kapeller, Schmidt and Hartung1999). Thus, greater baseline WMH volume may explain why females in the AT group exhibited greater WMH volume at trial completion.
Moreover, females in the AT group did not show improved functional cardiovascular capacity; as such, the physiological health benefits of AT may not have been attained. Higher levels of physical fitness have greater therapeutic benefits, particularly for the management of vascular risk factors (Lakka & Laaksonen, Reference Lakka and Laaksonen2007). Therefore, AT may need to be performed at a level that can modify vascular risks or the endothelium to impact WMH progression. Proper endothelial function is necessary in vascular homeostasis, and endothelial dysfunction contributes to disease states including small vessel disease (Bolduc, Thorin-Trescases, & Thorin, Reference Bolduc, Thorin-Trescases and Thorin2013; Lucas, Cotter, Brassard, & Bailey, Reference Lucas, Cotter, Brassard and Bailey2015). Specifically, the endothelium is important for regulating vascular tone by balancing the production of vasodilators, such as nitric oxide (Fernando et al., Reference Fernando, Simpson, Matthews, Brayne, Lewis and Barber2006). Endothelium nitric oxide signaling plays a key role in local cerebral blood flow regulation, and studies have found that participants with more WMHs display reduced cerebral blood flow (Markus, Allan, & Ebmeier, Reference Markus, Allan, Ebmeier, Pantoni, Gorelick and Gorelick2014). Many factors can regulate the release of nitric oxide, including AT. A study by Goto and colleagues (2003) found changes in endothelium-dependent forearm vasodilation after moderate intensity AT (50% of VO2 max) but not in low (25% of VO2 max) or high (75% of VO2 max) intensity AT. The authors postulated that high intensity AT increased oxidative stress, which may have abrogated any improvements in vascular function. Low intensity AT may fall below a given threshold for improvement in endothelial function (Goto et al., Reference Goto, Higashi, Kimura, Noma, Hara and Nakagawa2003). Therefore, AT intensity is an important factor in endothelium-dependent nitric oxide release. Because the females of this sub-study did not exhibit improved functional cardiovascular capacity, any potential benefits of AT on WMHs may have been attenuated.
Within the AT group, males displayed significantly less WMH progression than females at trial completion, despite having similar WMH volume at baseline, which suggests that AT may be more effective at mitigating WMH progression in males than in females. Current literature indicates that males and females respond differently to AT. Several studies report sex-specific hemodynamic responses to exercise, with more beneficial effects for men than women (Casey, Pierce, Howe, Mering, & Braith, Reference Casey, Pierce, Howe, Mering and Braith2007; Pierce, Eskurza, Walker, Fay, & Seals, Reference Pierce, Eskurza, Walker, Fay and Seals2011). Aerobic activity has been shown to prevent or mitigate age-related endothelial dysfunction in older men (Pierce et al., Reference Pierce, Eskurza, Walker, Fay and Seals2011), but this has not been consistently shown in older women (Casey et al., Reference Casey, Pierce, Howe, Mering and Braith2007). One study found that AT increased flow-mediated dilation in the brachial artery of older men, but not in older post-menopausal women (Pierce et al., Reference Pierce, Eskurza, Walker, Fay and Seals2011). This is supported by another study that reported that neither AT or resistance training had an effect on brachial flow-mediated dilation in sedentary, normotensive, post-menopausal women (Casey et al., Reference Casey, Pierce, Howe, Mering and Braith2007). Together, these studies suggest that the physiological effects of AT may be more potent in males than in females.
The results from our study should be evaluated within the context of its limitations. First, as an exploratory sub-study, our sample size was limited. Second, WMHs are considered an end-stage manifestation of small vessel disease; therefore, the inclusion of imaging techniques such as diffusion tensor imaging tractography or myelin water imaging may be more sensitive to detecting white matter changes. Third, females in the AT group did not show improved cardiovascular capacity as measured by the 6MWT; therefore, our study is limited in determining the efficacy of AT in mitigating WMH progression in women. The frequency, intensity, and duration of our AT intervention is similar to those in studies that have found an effect of AT in healthy older adults, but studies in people with SIVCI may need to implement AT at a higher intensity or longer duration to effectively demonstrate changes on MRI. Currently, there are no exercise recommendations for people with small vessel disease; however, we know that hypertension is most strongly associated with WMH progression (Prins & Scheltens, Reference Prins and Scheltens2015). The American College of Sports Medicine recommends aerobic exercise for a minimum of 3 days per week for 30–60 minutes at 40–70 per cent of heart rate reserve, but for optimal results, exercise should be performed daily. In addition, the American College of Sports Medicine recommends resistance training activities twice a week, focusing on lower weights but higher number of repetitions (8–12 per set) (American College of Sports Medicine, 2013). The Study of Mental Activity and Resistance Training (SMART) Trial, conducted in people with mild cognitive impairment, reported that high-intensity progressive resistance training resulted in a modest regression of WMHs in periventricular and parietal zones, whereas non-progressive resistance training groups displayed WMH progression (these results did not survive whole-brain correction) (Suo et al., Reference Suo, Singh, Gates, Wen, Sachdev and Brodaty2016). This is supported by another RCT that found that resistance training reduced WMH progression over 12 months in community dwelling older women (Bolandzadeh et al., Reference Bolandzadeh, Tam, Handy, Nagamatsu, Hsu and Davis2015). Therefore, future studies should consider combining both aerobic and resistance training. In addition, larger trials assessing the effect of physical activity on delaying WMH progression, such as the Australian Imaging Biomarkers and Lifestyle Flagship Study of Aging (AIBL) Active Trial (Cyarto et al., Reference Cyarto, Lautenschlager, Desmond, Ames, Szoeke and Salvado2012) and the ExCersion-Vascular Cognitive Impairment (VCI) study (Leeuwis et al., Reference Leeuwis, Hooghiemstra, Amier, Ferro, Franken and Nijveldt2017), may consider potential sex differences to more fully elucidate the efficacy of exercise in mitigating white matter changes. The results of our exploratory study provide the basis for the development of future exercise prescriptions and draw attention to the potential role of biological sex as a modifier in WMH progression.